Figure 2.
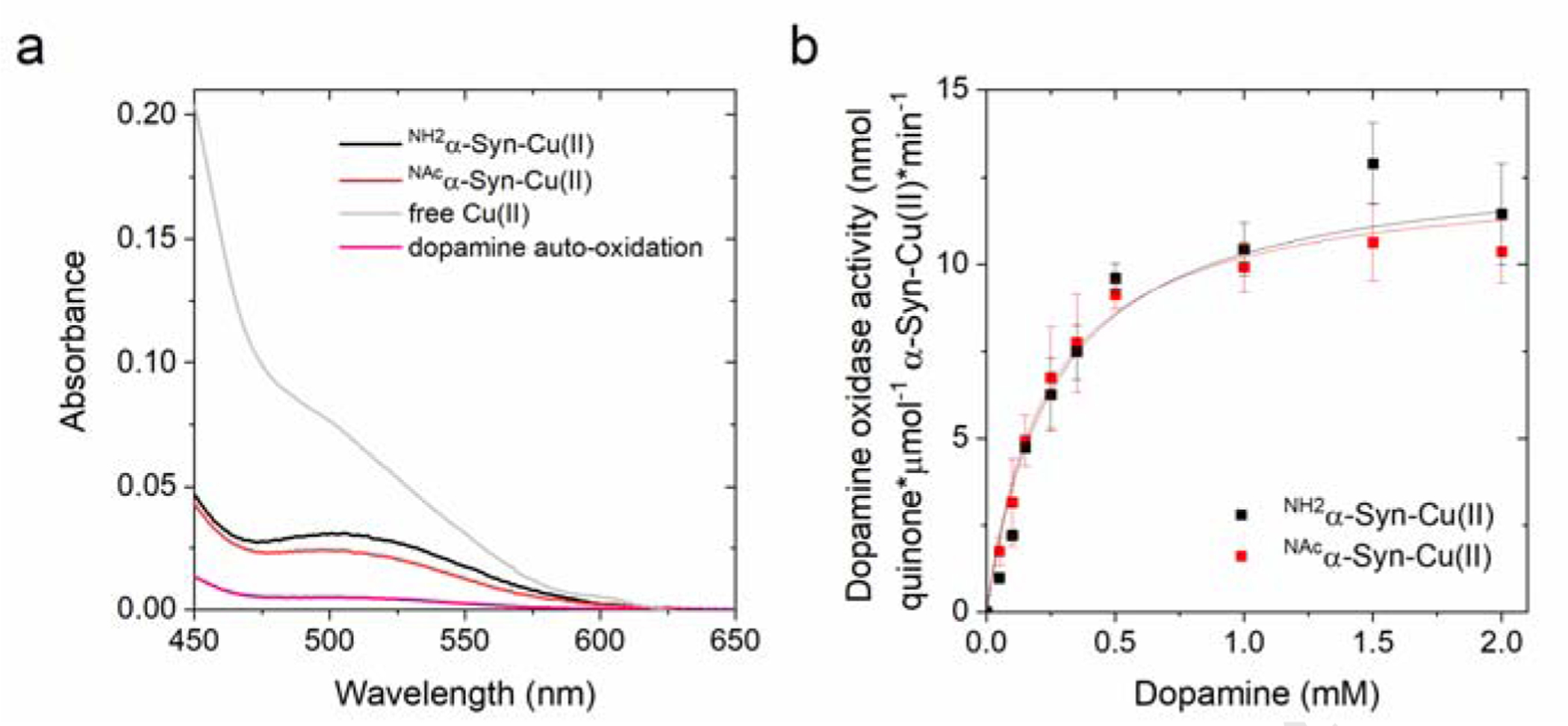
(a) Absorption spectra recorded after the reaction of NH2α-Syn-Cu(II) (10 μM; black), NAcα-Syn-Cu(II) (10 μM; red), or free Cu(II) (10 μM; gray) with dopamine (1 mM) in 20 mM N-ethylmorpholine/100 mM NaCl, pH 7.4 at 25°C, in the presence of MBTH (2 mM). Auto-oxidation of dopamine (1 mM) is plotted in pink. (b) Concentration-dependent dopamine oxidase activity of NH2α-Syn-Cu(II) (10 μM; black) or NAcα-Syn-Cu(II) (10 μM; red) in 20 mM N-ethylmorpholine/100 mM NaCl, pH 7.4, determined using MBTH (2 mM) to quantify the dopamine ortho-quinone formed after 120 s reaction (25°C).
