Figure 3.
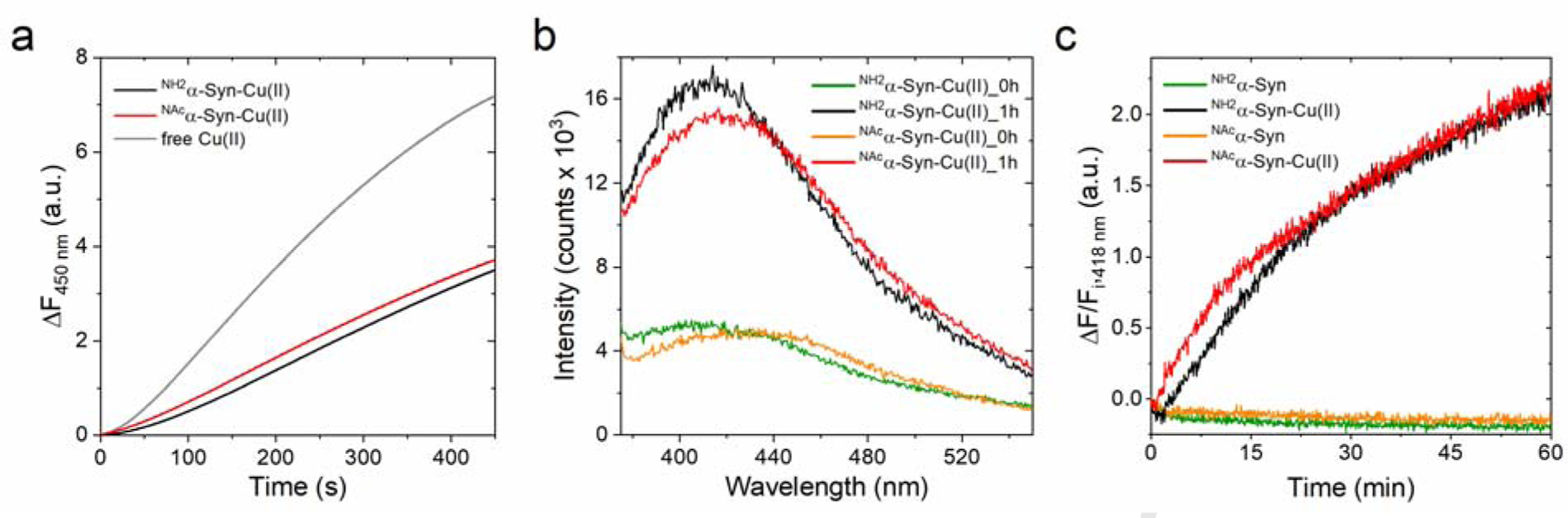
(a) Ascorbate-driven hydroxyl radical production by Cu(II) (5 μM; gray), NH2α-Syn-Cu(II) (5 μM; black), or NAcα-Syn-Cu(II) (5 μM; red) in the presence of ascorbate (600 μM) and 3-CCA (400 μM), determined by monitoring the formation of the fluorescent product 7-OH-CCA for 450 s at 37°C (λex=395 nm; λem=450 nm). (b) Dityrosine emission spectra (λex=325 nm) recorded forNH2α-Syn-Cu(II) (10 μM; green and black) and NAcα-Syn (10 μM; orange and red) before and after 1-h reaction with 1 mM ascorbate (37°C). (c) Kinetic traces monitoring dityrosine formation at 418 nm (λex=325 nm) in NH2α-Syn (green), NH2α-Syn-Cu(II) (black), NAcα-Syn (orange), and NAcα-Syn-Cu(II) (red). Dityrosine formation is reported as the difference between final and initial fluorescence at 418 nm (ΔF) over the initial fluorescence (Fi).
