Figure 6. Enhanced function of second-generation cam16-wt15–33 TriKE against AML targets in vivo.
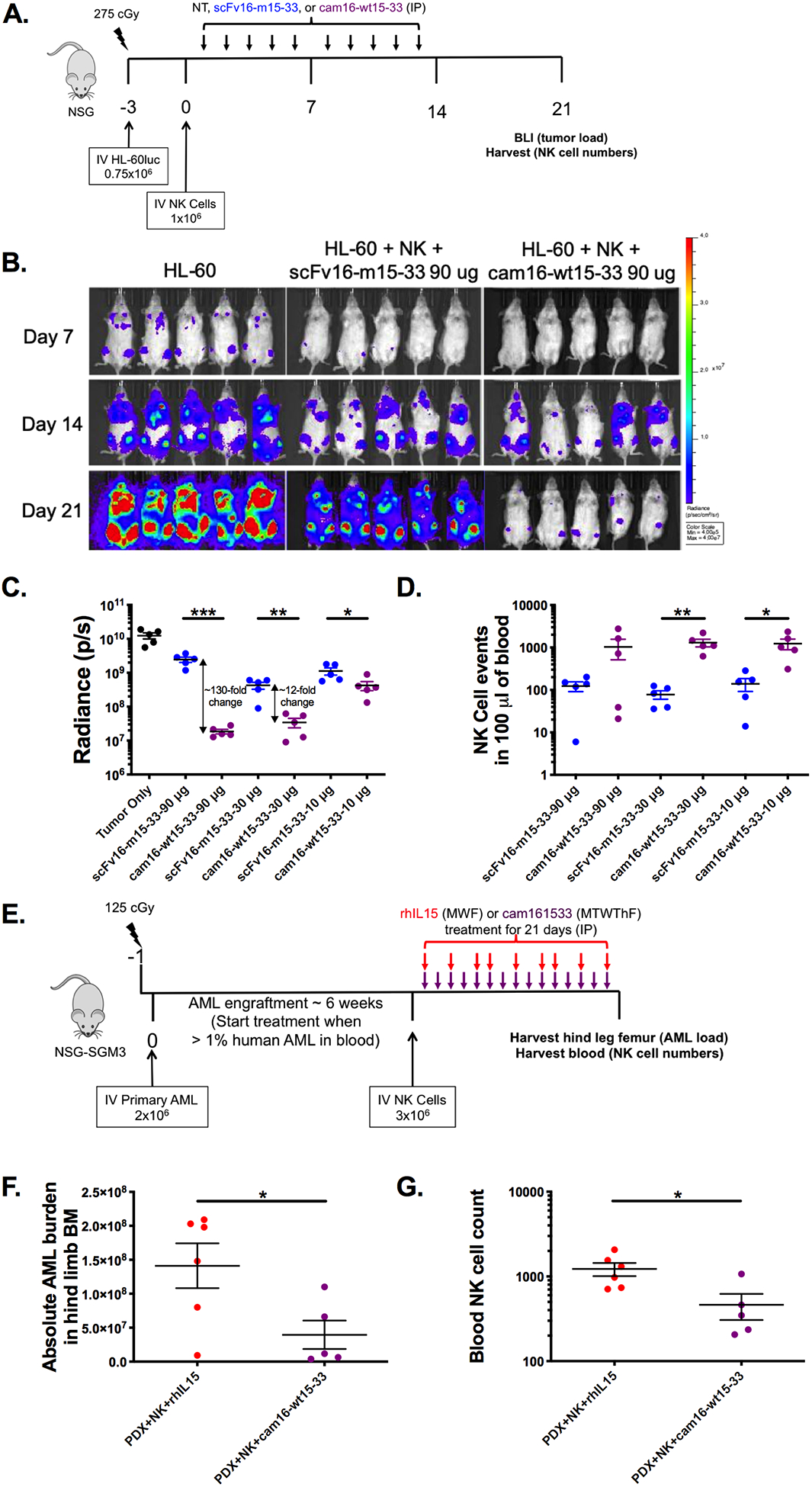
(a) Diagram of HL-60/human NK cell xenogneic NSG model for evaluation of TriKE activity. Mice were treated IP with noted concentrations of scFv16-m15–33 (blue) or cam16-wt15–33 (purple) 5 times weekly for 2 weeks. At days 7, 14, and 21, tumor load was assessed by bioluminescent imaging, and blood collected to evaluate NK cell expansion at day 21. (b) Image of individual mouse radiance on 90 μg treatment group over the course of the experiment. (c) Summary of tumor radiance on all treatment groups (90, 30, and 10 μg per injection groups), and (d) NK cell numbers at day 21 (N=5/group, representative of two experiments). (e) Diagram of AML patient-derived xenograft (PDX) model used to compare the second-generation cam16-wt15–33 TriKE to rhIL15. Upon AML engraftment, mice were injected with NK cells and treatments, and tissues were harvested 3 weeks later for evaluation of (f) AML blasts (CD45+CD33+) numbers in bone marrow and (g) NK cell numbers in a 100 μL of blood (N=5–6, representative of two experiments). Unpaired T test was used to calculate statistical differences. Bars show mean±SEM and statistical significance as *P<0.05, **P< 0.01, ***P<0.001, and ****P<0.0001.
