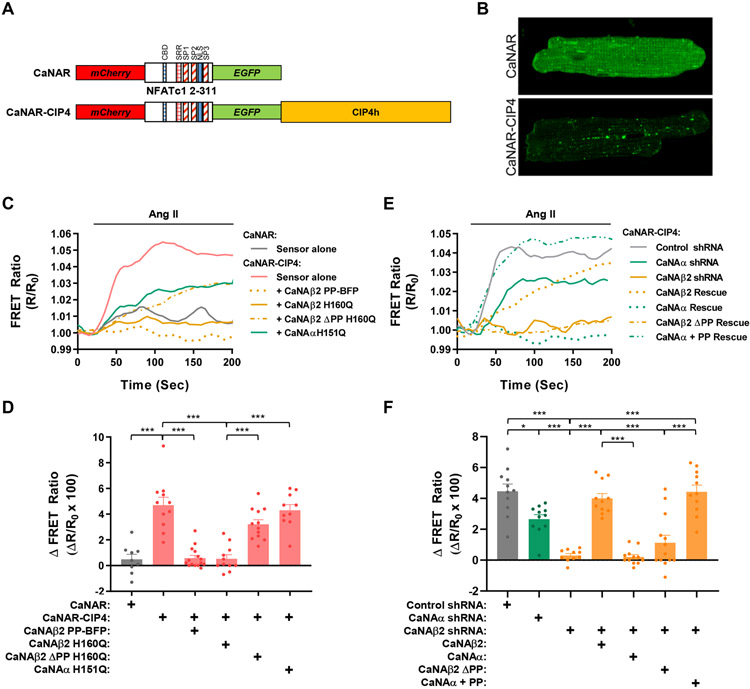
Figure 5. Polyproline-dependent compartmentation of CaNAβ activity.
A. Design of EGFP-mCherry calcineurin activity FRET sensors. CBD - CaN-binding PxIxIT domain; SRR, SP1, SP2 and SP3 - serine-rich domains subject to calcineurin dephosphorylation; NLS, nuclear localization signal (apparently inactive in sensor). B. Myocytes were infected with adenovirus expressing CaNAR or CaNAR-CIP4 FRET sensors and imaged by confocal microscopy. EGFP channels shown. C,D. Myocytes expressing CaNAR-CIP4 with or without CaNA mutant proteins or CaNAR alone were stimulated with Ang II (100 nmol/L) to induce calcineurin activity. Representative tracings and amplitude change for FRET ratio (R/R0) are shown. E,F. Myocytes expressing CaNAR-CIP4, shRNA, and CaNA mutant proteins were stimulated with Ang II (100 nmol/L). Bar graphs show results from individual tracings and mean ± s.e.m. Data passed Anderson-Darling (A2*) normality test and were analyzed by 1-way ANOVA with Tukey post-hoc testing. *p ≤ 0.05; ***p ≤ 0.001.