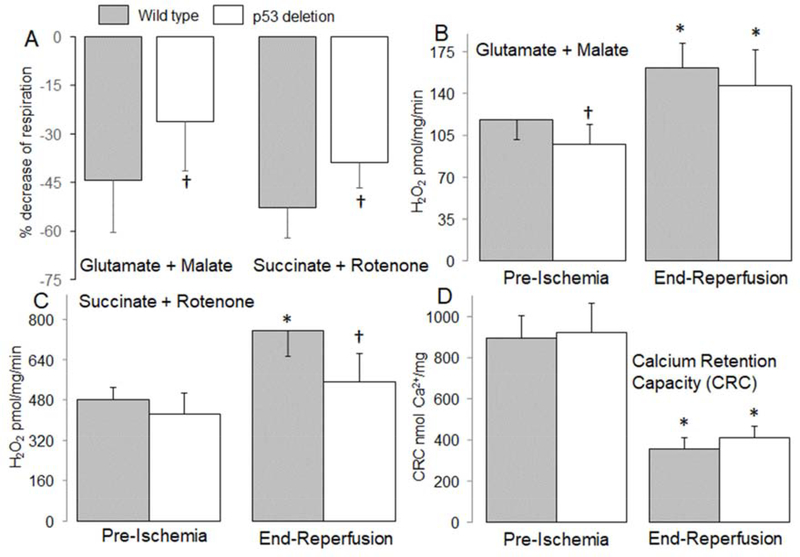
Figure 2. Deletion of p53 improved oxidative phosphorylation in mouse mitochondria following ischemia-reperfusion.
Ischemia-reperfusion decreased the dinitrophenol (DNP)-uncoupled rate of respiration in mitochondria isolated from wildtype mice oxidizing complex I and II substrates. The baseline rate of oxidation in wildtype [Mean ± SD: complex I (253 ± 57) and complex II (606 ± 41)] and in p53 deletion [Mean ± SD, complex I (243 ± 64) and complex II (562 ± 45)] mice is given. The percentage decrease in respiration during reperfusion was attenuated in p53 deletion mice (Panel A). Compared to wild type mice, H2O2 generation was decreased in p53 deletion before ischemia with complex I substrate (Panel B). The generation of H2O2 was increased in mitochondria isolated from both wild type and p53 deletion following reperfusion using complex I substrate (Panel B). Deletion of p53 decreased H2O2 generation during reperfusion when succinate + rotenone was used as complex II substrate compared to wild type mice (Panel C). Ischemia-reperfusion decreased the calcium retention capacity (CRC) in mitochondria isolated from both wild type and p53 deletion mice compared to mitochondria from non-ischemic hearts. Deletion of p53 did not significantly improve the CRC in mitochondria isolated following ischemia and 30 min. reperfusion compared to wild type controls (Panel D). Mean ± SD.
* p<0.05 vs. corresponding pre-ischemia. † p<0.05 vs. corresponding wild type hearts. n=6–8 in each group.