Abstract
The purpose of this study was to examine the melanoma targeting and imaging properties of 99mTc(CO)3-NOTA-GGNle-CycMSHhex {1,4,7-triazacyclononane-1,4,7-triyl-triacetic acid-GlyGlyNle-c[Asp-His-DPhe-Arg-Trp-Lys]-CONH2} and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex {1,4,7-triazacyclononane,1-gluteric acid-4,7-acetic acid-GlyGlyNle-c[Asp-His-DPhe-Arg-Trp-Lys]-CONH2} on B16/F10 melanoma-bearing C57 mice to demonstrate the feasibility of NOTA/NODAGA as metal chelators for 99mTc(CO)3+ radiolabeling.
Methods:
NOTA/NODAGA-GGNle-CycMSHhex were synthesized using fluorenylmethoxycarbonyl (Fmoc) chemistry. The melanocortin-1 (MC1) receptor binding affinities of the peptides were determined on B16/F10 melanoma cells. The biodistribution of 99mTc(CO)3-NOTA-GGNle-CycMSHhex and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex were determined on B16/F10 melanoma-bearing C57 mice at 2 h post-injection to select a lead peptide for further evaluation. The melanoma targeting and imaging properties of 99mTc(CO)3-NOTA-GGNle-CycMSHhex and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex were determined on B16/F10 melanoma-bearing C57 mice.
Results:
The IC50 values of NOTA/NODAGA-GGNle-CycMSHhex were 0.9 ± 0.1 and 0.8 ± 0.05 nM on B16/F10 cells. 99mTc(CO)3-NOTA-GGNle-CycMSHhex and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex were readily prepared using via [99mTc(CO)3(OH2)3]+ intermediate, and displayed MC1R-specific binding on B16/F10 cells. 99mTc(CO)3-NOTA-GGNle-CycMSHhex was further evaluated as a lead peptide because of its higher tumor uptake (19.76 ± 3.62 %ID/g) and lower kidney uptake (1.59 ± 0.52 %ID/g) at 2 h post-injection than 99mTc(CO)3-NODAGA-GGNle-CycMSHhex. The B16/F10 melanoma uptake of 99mTc(CO)3-NOTA-GGNle-CycMSHhex was 16.07 ± 4.47, 19.76 ± 3.62, 11.30 ± 2.81 and 3.16 ± 2.28% ID/g at 0.5, 2, 4 and 24 h post-injection, respectively. 99mTc(CO)3-NOTA-GGNle-CycMSHhex showed high tumor to normal organ uptake ratios after 2 h post-injection. The B16/F10 melanoma lesions were clearly visualized by SPECT/CT using 99mTc(CO)3-NOTA-GGNle-CycMSHhex as an imaging probe at 2 h post-injection.
Conclusions:
High tumor uptake, low kidney uptake and fast urinary clearance of 99mTc(CO)3-NOTA-GGNle-CycMSHhex highlighted its potential for melanoma imaging and facilitated the evaluation of 188Re(CO)3-NOTA-GGNle-CycMSHhex for melanoma therapy.
Keywords: 99mTc(CO)3-NOTA-GGNle-CycMSHhex, alpha-melanocyte-stimulating hormone, melanocortin-1 receptor, melanoma targeting, SPECT
Graphical Abstract
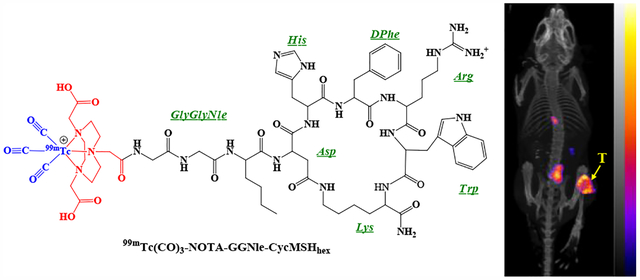
INTRODUCTION
Malignant melanoma is the most lethal form of skin cancer due to the extreme aggressiveness of melanoma metastasis. It is estimated that approximately 100,350 new cases and 6,850 fatalities will occur in the United States in 2020.1 Traditional overall survival of patients with metastatic melanoma is only 6–9 months. New molecular treatments including Vemurafenib (BRAF inhibitor), ipilimumab (targeting CTLA-4) and Nivolumab (PD-1 inhibitor) have demonstrated encouraging clinical benefits for metastatic melanoma patients by improving the overall survival by months.2–6 However, the treatments are still far from satisfactory because the 5-year survival is approximately 35% for patients with metastatic melanoma.7 Therefore, it is highly desirable to develop new therapeutic molecular approaches for metastatic melanoma.
We have been targeting melanocortin-1 receptors (MC1Rs) for melanoma imaging and therapy due to the over-expression of MC1Rs on both melanotic and amelanotic human melanoma samples.8, 9 Our first-in-human study underscored the clinical relevance of MC1R as an attractive molecular target for developing theranostic peptides for melanoma.10 Over the past several years, we have developed a novel class of radiolabeled lactam-cyclized α-melanocyte-stimulating hormone (α-MSH) peptides,11–20 taking advantage of the nanomolar MC1R binding affinity of key construct of GGNle-CycMSHhex {Gly-Gly-Nle-c[Asp-His-DPhe-Arg-Trp-Lys]-CONH2} and various radiometal chelators such as HYNIC, DOTA and NOTA for radiolabeling of theranostic radionuclides. For instance, HYNIC was used for 99mTc radiolabeling,15, 16 whereas DOTA and NOTA were utilized for radiolabeling of diagnostic 111In,11, 12 203Pb,18 67/68Ga,10, 13 64Cu14 and therapeutic 177Lu17 and 90Y.19 These radiolabeled α-MSH peptides generally yielded high melanoma uptake and rapid urinary clearance, highlighting their potential for melanoma imaging and therapy. For example, 111In-DOTA-GGNle-CycMSHhex and 177Lu-DOTA-GGNle-CycMSHhex displayed high tumor uptake of 19.05 ± 5.04 and 21.63 ± 6.27 %ID/g at 2 h post-injection, respectively.12, 17
Technetium-99m is an attractive single photon emission computed tomography (SPECT) radionuclide due to its 6-h half life, 140 keV gamma ray and high availability through a cost-effective 99Mo/99mTc generator. In terms of 99mTc-labeled lactam-cyclized α-MSH peptides, we have used mercaptoacetyltriglycine (MAG3)-GGNle-CycMSHhex, Ac-Cys-Gly-Gly-Gly (AcCG3)-GGNle-CycMSHhex to form stable complexes with 99mTc=O3+ core, and hydrazinonicotinamide (HYNIC)-GGNle-CycMSHhex to yield stable 99mTc-conjugates via [99mTc(CO)3(OH2)3]+ tricarbonyl intermediate and EDDA/tricine coligands. Interestingly, 99mTc-MAG3-GGNle-CycMSHhex and 99mTc-AcCG3-GGNle-CycMSHhex displayed 4.64 ± 1.06 and 9.76 ± 4.90 %ID/g on B16/F1 melanoma at 2 h post-injection.15 Although 99mTc(CO)3-HYNIC-GGNle-CycMSHhex exhibited comparable tumor uptake of 5.84 ± 1.26 %ID/g, it also showed high liver and kidney uptake of 38.11 ± 2.31 and 17.69 ± 4.06 %ID/g at 2 h post-injection, respectively.15 Meanwhile, 99mTc(EDDA)-HYNIC-GGNle-CycMSHhex exhibited higher tumor uptake (14.14 ± 4.90 %ID/g) and lower liver and renal uptake (0.52 ± 0.05 and 7.52 ± 0.96 %ID/g) than 99mTc(CO)3-HYNIC-GGNle-CycMSHhex at 2 h post-injection15. The tremendous differences in biodistribution properties of these above-mentioned 99mTc-CycMSHhex peptides suggested that the coordination of 99mTc core could potentially affect the tumor targeting and clearance properties of 99mTc-labeled lactam-cyclized α-MSH peptides on melanoma-bearing mice.
We demonstrated that 1,4,7-triazacyclononane-1,4,7-triyl-triacetic acid (NOTA)-GGNle-CycMSHhex could form stable hydrophilic complexes with 67Ga and 64Cu in our previous report.13, 14 Both 67Ga-NOTA-GGNle-CycMSHhex and 64Cu-NOTA-GGNle-CycMSHhex exhibited high melanoma uptake, highlighting their potential for SPECT and positron emission tomography (PET) imaging of melanoma.13, 14 Meanwhile, the tridentate NOTA and NODAGA could coordinate [99mTc(CO)3]+ tricarbonyl core via three N atoms to yield stable bombesin and somatostatin peptide conjugates for tumor targeting.20–24 Recently, an interesting report found that 99mTc(CO)3-NOTA-sst2-ANT exhibited 7 to 8-fold higher tumor uptake than 99mTc(CO)3-NODAGA-sst2-ANT on AR42J tumor-bearing mice.24 Thus we were interested whether and how this new coordination chemistry of [99mTc(CO)3]+ tricarbonyl core could affect the biodistribution properties of NOTA/NODAGA-conjugated lactam-cyclized α-MSH peptides. In this study, we synthesized NOTA/NODAGA-GGNle-CycMSHhex using fluorenylmethoxycarbonyl (Fmoc) chemistry, determined their MC1R binding affinities on B16/F10 melanoma cells, prepared their 99mTc(CO)3-conjugates using [99mTc(CO)3(OH2)3]+ tricarbonyl intermediate and examined their biodistribution and tumor targeting properties on B16/F10 melanoma-bearing C57 mice.
EXPERIMENTAL SECTION
Chemicals and reagents
Amino acid and resin were purchased from Advanced ChemTec Inc. (Louisville, KY) and Novabiochem (San Diego, CA). NOTA(OtBu)2 and NODAGA(OtBu)3 were purchased from CheMatech Inc. (Dijon, France) for peptide synthesis. 125I-Tyr2-[Nle4, D-Phe7]-α-MSH {125I-(Tyr2)-NDP-MSH} was obtained from PerkinElmer, Inc. (Waltham, MA) for in vitro binding assay. [99mTcO4]− was purchased from Cardinal Health (Denver, CO) and IsoLink kit was obtained from Mallinckrodt (St. Louis, MO) for 99mTc labeling. B16/F10 murine melanoma cells were received from American Type Culture Collection (Manassas, VA). All other chemicals used in this study were purchased from Thermo Fisher Scientific (Waltham, MA) and used without further purification.
Peptide synthesis
NOTA-GGNle-CycMSHhex and NODAGA-GGNle-CycMSHhex were synthesized using fluorenylmethoxycarbonyl (Fmoc) chemistry. Briefly, the linear intermediate scaffolds of NOTA(OtBu)2/NODAGA(OtBu)3-Nle-Gly-Gly-Asp(O-2-PhiPr)-His(Trt)-DPhe-Arg(Pbf)-Trp(Boc)-Lys(Mtt) were synthesized on Sieber amide resin by an Advanced ChemTech multiple-peptide synthesizer (Louisville, KY). Generally, 70 μmol of resin, 210 μmol of each Fmoc-protected amino acid and NOTA(OtBu)2/NODAGA(OtBu)3 were used for the synthesis. The protecting groups of Mtt and 2-phenylisopropyl were removed by 2.5% of trifluoroacetic acid (TFA) for peptide cyclization. The cyclization reaction was achieved on the resin by an overnight reaction at 25 °C in dimethylformamide (DMF) using benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium-hexafluorophosphate (PyBOP) as a coupling agent in the presence of N,N-diisopropylethylamine (DIPEA). All protecting groups were totally removed and the peptide was cleaved from the resin by treating with a mixture of trifluoroacetic acid (TFA), thioanisole, phenol, water, ethanedithiol and triisopropylsilane (87.5:2.5:2.5:2.5:2.5:2.5) for 2 h at 25 °C. The peptide was precipitated and washed with ice-cold ether four times, purified by RP-HPLC and characterized by LC-MS.
In vitro competitive binding assay
The MC1R binding affinities of NOTA/NODAGA-GGNle-CycMSHhex were determined on B16/F10 melanoma cells by in vitro competitive receptor binding assay. The receptor binding assay was replicated in triplicate. The B16/F10 cells (0.5×105 cells/well, n = 3) were incubated at room temperature (25 °C) for 2 h with approximately 30,000 counts per minute (cpm) of 125I-(Tyr2)-NDP-MSH in the presence of 10−12 to 10−5 M of the peptide in 0.3 mL of binding medium {Modified Eagle’s medium with 25 mM N-(2-hydroxyethyl)-piperazine-N’-(2-ethanesulfonic acid), pH 7.4, 0.2% bovine serum albumin (BSA), 0.3 mM 1,10-phenathroline}. The binding medium was aspirated after the incubation. The cells were rinsed twice with 0.5 mL of ice-cold pH 7.4, 0.2% BSA/0.01 M phosphate buffered saline (PBS) and lysed in 0.5 mL of 1 N NaOH for 5 min. The cells were harvested and measured in a Wallac 1480 automated gamma counter (PerkinElmer, NJ). The IC50 value was calculated using the Prism software (GraphPad Software, La Jolla, CA, USA).
Radiolabeling
99mTc(CO)3-NOTA-GGNle-CycMSHhex and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex were prepared using IsoLink kit according to our previous publication15 with modifications. Briefly, 2 mL of freshly eluted 99mTcO4− (~383 MBq) solution was added into an IsoLink kit, followed by heating at 100 °C for 20 min to yield [99mTc(CO)3(OH2)3]+ intermediate. The kit vial was removed from the water bath and allowed to cool down to room temperature. For each radiolabeling reaction, 60 μL of 1 M HCl was added into 0.7 mL of [99mTc(CO)3(OH2)3]+ (~126 MBq) solution in a reaction vial to adjust the pH to 7 first, then 50 μL of 1 mg/mL peptide aqueous solution was added into the reaction vial. After adjusting the pH to 5–6 using 0.5 M NH4OAc-buffered solution (pH 5.3), the reaction mixture was heated at 95 °C for 30 min and cooled down to room temperature.
The radiolabeled complexes were purified to single species by a Waters RP-HPLC (Milford, MA) on a Grace Vydac C-18 reverse phase analytical column (Deerfield, IL) using the following gradient at a 1 mL/min flowrate. The mobile phase consisted of solvent A (20 mM HCl aqueous solution) and solvent B (100% CH3CN). The gradient was initiated and kept at 78:22 A/B for 3 min followed by a linear gradient of 78:22 A/B to 68:32 A/B over 20 min. Then, the gradient was changed from 68:32 A/B to 10:90 A/B over 3 min followed by an additional 5 min at 10:90 A/B. Thereafter, the gradient was changed from 10:90 A/B to 78:22 A/B over 3 min. The purified peptide sample was purged with N2 gas for 15 min to remove the acetonitrile. The pH of the final solution was adjusted to 7.4 with 0.1 N NaOH and sterile saline.
Specific binding of 99mTc(CO)3-NOTA-GGNle-CycMSHhex and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex
The specific binding of 99mTc(CO)3-NOTA-GGNle-CycMSHhex and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex was determined on B16/F10 melanoma cells, respectively. Briefly, the B16/F10 cells (1×106 cells/tube, n = 3) were incubated at 25 °C for 2 h with approximately 0.22 MBq of either 99mTc(CO)3-NOTA-GGNle-CycMSHhex or 99mTc(CO)3-NODAGA-GGNle-CycMSHhex with or without 10 μg (6.07 nmol) of unlabeled [Nle4, D-Phe7]-α-MSH (NDP-MSH) in 0.3 mL of binding medium {Modified Eagle’s medium with 25 mM N-(2-hydroxyethyl)-piperazine-N’-(2-ethanesulfonic acid), pH 7.4, 0.2% bovine serum albumin (BSA), 0.3 mM 1,10-phenathroline}. After the incubation, the cells were rinsed twice with 0.5 mL of ice-cold pH 7.4, 0.2% BSA/0.01 M phosphate buffered saline (PBS), lysed with 0.5 mL of 1 M NaOH for 5 min, collected and measured in a Wallac 1480 automated gamma counter (PerkinElmer, NJ).
Biodistribution and melanoma imaging studies
All animal studies were conducted in compliance with Institutional Animal Care and Use Committee approval. Each C57 mouse was subcutaneously inoculated with 1×106 B16/F10 cells on the right flank to generate melanoma tumors. Ten days post inoculation, the tumor weights reached approximately 0.2 g and the melanoma-bearing mice were used for biodistribution and imaging studies. The biodistribution properties of 99mTc(CO)3-NOTA-GGNle-CycMSHhex and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex were determined on B16/F10 melanoma-bearing C57 mice at 2 h post-injection first to select one peptide for further evaluation. Because 99mTc(CO)3-NOTA-GGNle-CycMSHhex displayed more favorable tumor targeting property than 99mTc(CO)3-NODAGA-GGNle-CycMSHhex, the full biodistribution of 99mTc(CO)3-NOTA-GGNle-CycMSHhex was further examined on B16/F10 melanoma-bearing C57 mice.
For full biodistribution of 99mTc(CO)3-NOTA-GGNle-CycMSHhex, each melanoma-bearing mouse was injected with 0.037 MBq of 99mTc(CO)3-NOTA-GGNle-CycMSHhex via the tail vein. The specificity of the tumor uptake of 99mTc(CO)3-NOTA-GGNle-CycMSHhex was determined by co-injecting 10 μg (6.07 nmol) of unlabeled NDP-MSH which is a linear α-MSH peptide analogue with sub-nanomolar MC1R binding affinity. Mice were sacrificed at 0.5, 2, 4 and 24 h post-injection, and tumors and organs of interest were harvested, weighed and counted. Blood values were taken as 6.5% of the whole-body weight.
The melanoma imaging property of 99mTc(CO)3-NOTA-GGNle-CycMSHhex was examined on B16/F10 melanoma-bearing C57 mice and compared with that of 99mTc(CO)3-NODAGA-GGNle-CycMSHhex. Approximately 11.1 MBq of 99mTc(CO)3-NOTA-GGNle-CycMSHhex or 99mTc(CO)3-NODAGA-GGNle-CycMSHhex was injected in B16/F10 melanoma-bearing C57 mice via the tail veins for melanoma imaging. Each mouse was sacrificed at 2 h post-injection for small animal SPECT/CT (nanoScan SPECT/CT, Mediso) imaging. The CT scan was immediately followed by the whole-body SPECT scan. Reconstructed SPECT and CT data were visualized and co-registered using VivoQuant (inviCRO, Boston, MA).
Statistical analysis
Statistical analysis was performed using the Student’s t-test for unpaired data. A 95% confidence level was chosen to determine the significance of difference in cellular binding, in tumor, blood, liver and kidney uptake between 99mTc(CO)3-NOTA-GGNle-CycMSHhex and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex, in tumor and renal uptake of 99mTc(CO)3-NOTA-GGNle-CycMSHhex with or without NDP-MSH co-injection. The differences at the 95% confidence level (p<0.05) were considered significant.
RESULTS
NOTA/NODAGA-GGNle-CycMSHhex (Figure 1) were synthesized and purified by reverse phase high pressure liquid chromatography (RP-HPLC). After the HPLC purification, NOTA/NODAGA-GGNle-CycMSHhex displayed greater than 90% purity. The identities of NOTA/NODAGA-GGNle-CycMSHhex was confirmed by electrospray ionization mass spectrometry. The calculated and found molecular weights of NOTA/NODAGA-GGNle-CycMSHhex were 1381, 1381, 1453 and 1453, respectively. The IC50 values of NOTA/NODAGA-GGNle-CycMSHhex were 0.9 ± 0.1 and 0.8 ± 0.05 nM on B16/F10 melanoma cells (Figure 2).
Figure 1.
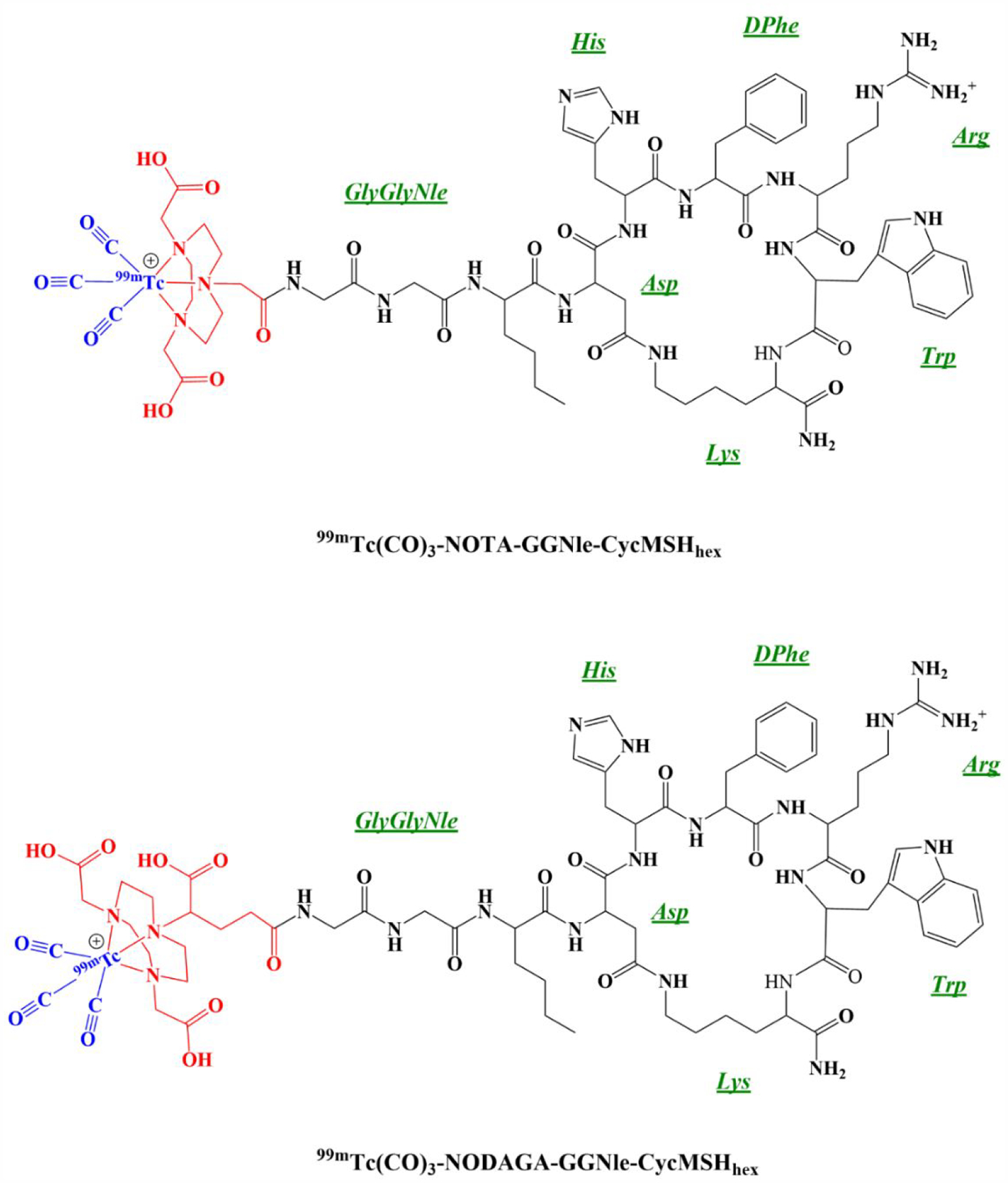
The proposed schematic structures of 99mTc(CO)3-NOTA-GGNle-CycMSHhex and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex.
Figure 2.
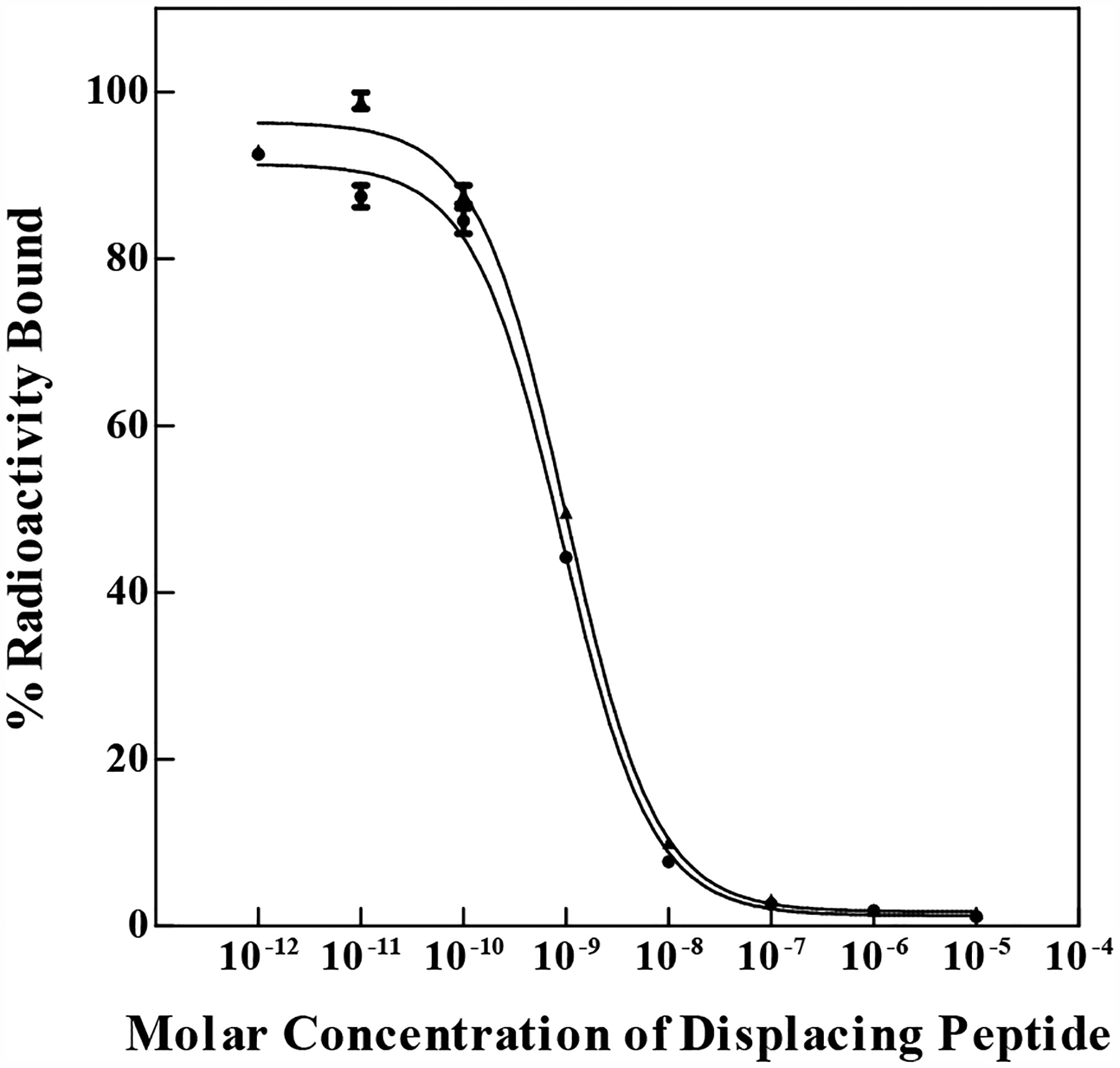
In vitro competitive binding curves of NOTA-GGNle-CycMSHhex (●) and NODAGA-GGNle-CycMSHhex (▲). The IC50 value of NOTA-GGNle-CycMSHhex and NODAGA-GGNle-CycMSHhex was 0.9 ± 0.1 and 0.8 ± 0.05 nM on B10/F10 melanoma cells, respectively.
99mTc(CO)3-NOTA-GGNle-CycMSH and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex were readily prepared using IsoLink kit and collected by HPLC. The radiochemical purity of 99mTc(CO)3-NOTA-GGNle-CycMSHhex and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex was greater than 95%, respectively (Figure 3). Both 99mTc(CO)3-NOTA-GGNle-CycMSHhex and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex displayed receptor-mediated binding on B16/F10 melanoma cells. Approximately 75% of cellular uptake of 99mTc(CO)3-NOTA-GGNle-CycMSHhex was blocked by peptide blockade, whereas 76% of cellular uptake of 99mTc(CO)3-NODAGA-GGNle-CycMSHhex was blocked by peptide blockade (Figure 3). Interestingly, 99mTc(CO)3-NOTA-GGNle-CycMSHhex exhibited higher receptor binding than 99mTc(CO)3-NODAGA-GGNle-CycMSHhex on B16/F10 cells.
Figure 3.
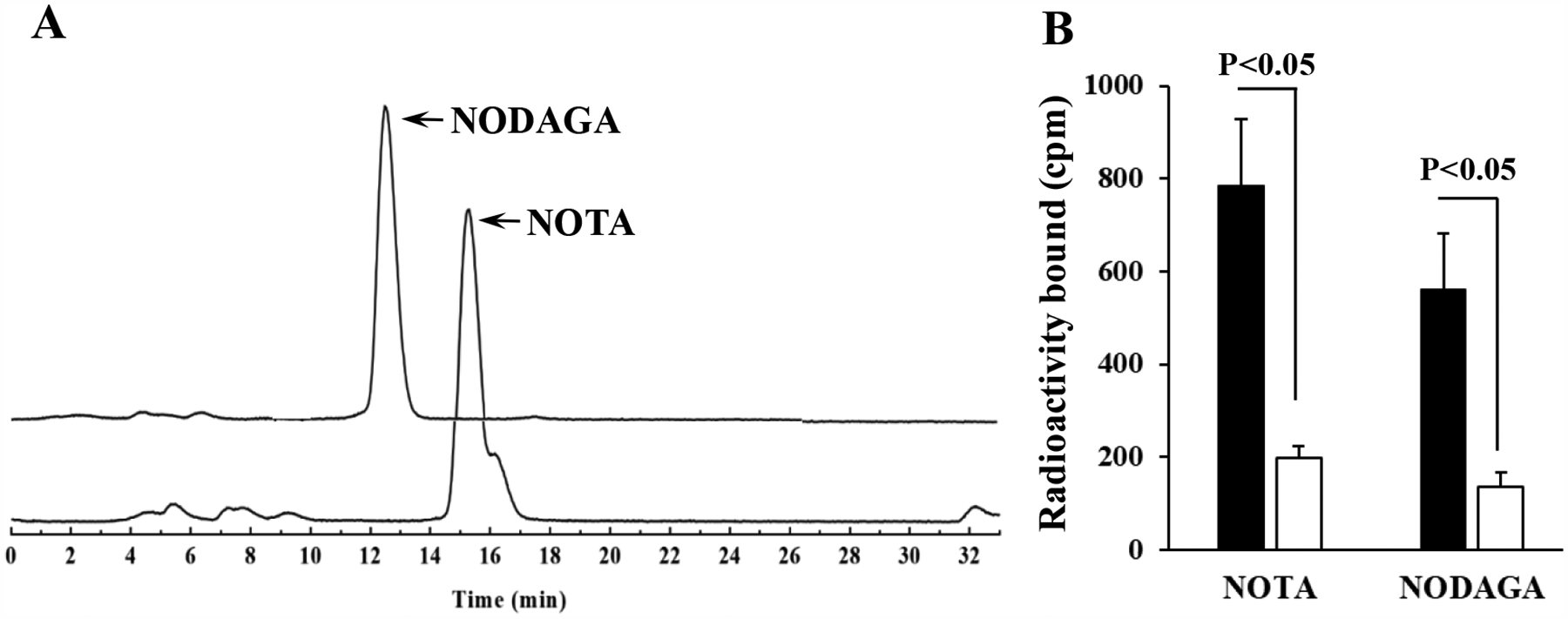
Radioactive HPLC profiles (A) of 99mTc(CO)3-NOTA-GGNle-CycMSHhex (NOTA) and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex (NODAGA). The retention time of 99mTc(CO)3-NOTA-GGNle-CycMSHhex and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex was 15.6 and 12.8 min, respectively. Specific binding (B) of 99mTc(CO)3-NOTA-GGNle-CycMSHhex and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex on B16/F10 melanoma cells with (white) and without (black) peptide blockade.
The biodistribution comparison between 99mTc(CO)3-NOTA-GGNle-CycMSHhex and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex at 2 h post-injection is presented in Table 1. The tumor uptake of 99mTc(CO)3-NOTA-GGNle-CycMSHhex was higher than that of 99mTc(CO)3-NODAGA-GGNle-CycMSHhex. The tumor uptake of 99mTc(CO)3-NOTAGGNle-CycMSHhex and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex was 19.76 ± 3.62 and 7.19 ± 1.80 %ID/g at 2 h post-injection, respectively. Although the uptake of 99mTc(CO)3-NOTA-GGNle-CycMSHhex in blood and liver was higher than that of 99mTc(CO)3-NODAGA-GGNle-CycMSHhex, the uptake of 99mTc(CO)3-NOTA-GGNle-CycMSHhex in other normal organs was similar to that of 99mTc(CO)3-NODAGA-GGNle-CycMSHhex except for kidneys. Interestingly, the renal uptake of 99mTc(CO)3-NOTAGGNle-CycMSHhex was much lower than that of 99mTc(CO)3-NODAGA-GGNle-CycMSHhex by 5-fold. The renal uptake of 99mTc(CO)3-NOTA-GGNle-CycMSHhex was as low as 1.59 ± 0.52 %ID/g at 2 h post-injection. Therefore, the full biodistribution of 99mTc(CO)3-NOTA-GGNle-CycMSHhex at 0.5, 4 and 24 h post-injection was further evaluated in this study.
Table 1.
Biodistribution comparison between 99mTc(CO)3-NOTA-GGNle-CycMSHhex (NOTA) and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex (NODAGA) on B16/F10 melanoma-bearing C57 mice at 2 h post-injection. The data were presented as percent injected dose/gram or as percent injected dose (Mean ± SD, n = 4)
| Tissues | NOTA | NODAGA |
|---|---|---|
| Percent injected dose/gram (%ID/g) | ||
| Tumor | 19.76 ± 3.62 | 7.19 ± 1.80* |
| Brain | 0.03 ± 0.01 | 0.02 ± 0.01 |
| Blood | 2.19 ± 0.73 | 0.55 ± 0.28* |
| Heart | 0.11 ± 0.07 | 0.15 ± 0.02 |
| Lung | 0.28 ± 0.17 | 0.30 ± 0.13 |
| Liver | 1.57 ± 0.32 | 0.34 ± 0.03* |
| Spleen | 0.38 ± 0.20 | 0.13 ± 0.03 |
| Stomach | 0.50 ± 0.37 | 0.31 ± 0.09 |
| Kidneys | 1.59 ± 0.52 | 8.66 ± 2.59* |
| Muscle | 0.09 ± 0.06 | 0.09 ± 0.03 |
| Pancreas | 0.11 ± 0.06 | 0.10 ± 0.07 |
| Bone | 0.29 ± 0.09 | 0.20 ± 0.06 |
| Skin | 0.35 ± 0.14 | 0.29 ± 0.03 |
| Percent injected dose (%ID) | ||
| Intestines | 2.95 ± 0.25 | 0.56 ± 0.15 |
| Urine | 89.50 ± 2.10 | 92.02 ± 1.36 |
| Uptake ratio of tumor/normal tissue | ||
| Tumor/blood | 9.02 | 13.07 |
| Tumor/kidney | 12.43 | 0.83 |
| Tumor/lung | 70.57 | 23.97 |
| Tumor/liver | 12.59 | 21.15 |
| Tumor/muscle | 219.56 | 79.89 |
| Tumor/skin | 56.46 | 24.79 |
p<0.05 for determining significance of differences in tumor, blood, liver and kidney uptake between 99mTc(CO)3-NOTA-GGNle-CycMSHhex and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex.
The full biodistribution results of 99mTc(CO)3-NOTA-GGNle-CycMSHhex are presented in Table 2. The accumulation of 99mTc(CO)3-NOTA-GGNle-CycMSHhex in the tumor was rapid and high, with 16.07 ± 4.47 %ID/g at 0.5 h post-injection. The tumor uptake of 99mTc(CO)3-NOTA-GGNle-CycMSHhex was 19.76 ± 3.62 and 11.30 ± 2.81 %ID/g at 2 and 4 h post-injection, and gradually decreased to 3.16 ± 2.28 %ID/g at 24 h post-injection. Approximately 96% of the tumor uptake was blocked at 2 h post-injection, demonstrating that the tumor uptake was MC1R-medicated. The blood uptake was 2.19 ± 0.73 %ID/g at 2 h post-injection and quickly reduced to 0.11 ± 0.06 %ID/g at 4 h post-injection. Interestingly, the renal uptake dramatically decreased from 7.16 ± 0.79 %ID/g at 0.5 h post-injection to 1.59 ± 0.52 %ID/g at 2 h post-injection, and eventually reached 0.81 ± 0.21 and 0.23 ± 0.19 %ID/g at 4 and 24 h post-injection. The peptide blockade didn’t decrease the renal uptake, indicating that the renal uptake wasn’t MC1R-specific. 99mTc(CO)3-NOTA-GGNle-CycMSHhex displayed a rapid urinary excretion, approximately 90% of the injected activity being cleared at 2 h post-injection. Fast clearance from normal organs resulted in high tumor to normal organ uptake rations as early as 0.5 h post-injection.
Table 2.
Biodistribution of 99mTc(CO)3-NOTA-GGNle-CycMSHhex on B16/F10 melanoma-bearing C57 mice. The data were presented as percent injected dose/gram or as percent injected dose (Mean ± SD, n = 4)
| Tissues | 0.5 h | 2 ha | 4 h | 24 h | 2 h NDP blockade |
|---|---|---|---|---|---|
| Percent injected dose/gram (%ID/g) | |||||
| Tumor | 16.07 ± 4.47 | 19.76 ± 3.62 | 11.30 ± 2.81 | 3.16 ± 2.28 | 0.80 ± 0.11* |
| Brain | 0.13 ± 0.02 | 0.03 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.04 ±.0.03 |
| Blood | 1.44 ± 0.33 | 2.19 ± 0.73 | 0.11 ± 0.06 | 0.03 ± 0.05 | 0.22 ± 0.18 |
| Heart | 0.74 ± 0.20 | 0.11 ± 0.07 | 0.07 ± 0.03 | 0.08 ± 0.07 | 0.17 ± 0.05 |
| Lung | 2.11 ± 0.65 | 0.28 ± 0.17 | 0.18 ± 0.05 | 0.03 ± 0.03 | 0.44 ± 0.26 |
| Liver | 2.32 ± 0.20 | 1.57 ± 0.32 | 1.43 ± 0.31 | 0.53 ± 0.17 | 4.32 ± 1.34 |
| Spleen | 0.76 ± 0.48 | 0.38 ± 0.20 | 0.11 ± 0.05 | 0.19 ± 0.25 | 0.78 ± 0.44 |
| Stomach | 1.11 ± 0.42 | 0.50 ± 0.37 | 0.25 ± 0.06 | 0.06 ± 0.07 | 1.02 ± 0.36 |
| Kidneys | 7.16 ± 0.79 | 1.59 ± 0.52 | 0.81 ± 0.21 | 0.23 ± 0.19 | 3.07 ± 0.61* |
| Muscle | 0.19 ± 0.06 | 0.09 ± 0.06 | 0.01 ± 0.01 | 0.03 ± 0.04 | 0.08 ± 0.08 |
| Pancreas | 0.29 ± 0.10 | 0.11 ± 0.06 | 0.01 ± 0.01 | 0.03 ± 0.05 | 0.14 ± 0.17 |
| Bone | 0.82 ± 0.41 | 0.29 ± 0.09 | 0.07 ± 0.06 | 0.01 ± 0.00 | 0.16 ± 0.05 |
| Skin | 1.89 ± 0.44 | 0.35 ± 0.14 | 0.07 ± 0.07 | 0.12 ± 0.12 | 0.12 ± 0.18 |
| Percent injected dose (%ID) | |||||
| Intestines | 2.41 ± 0.31 | 2.95 ± 0.25 | 2.60 ± 0.28 | 0.40 ± 0.40 | 4.18 ± 1.90 |
| Urine | 80.18 ± 2.97 | 89.50 ± 2.10 | 92.88 ± 0.42 | 97.76 ± 0.56 | 89.26 ± 2.78 |
| Uptake ratio of tumor/normal tissue | |||||
| Tumor/blood | 11.16 | 9.02 | 102.64 | 105.33 | 3.64 |
| Tumor/kidney | 2.24 | 12.43 | 13.94 | 13.74 | 0.26 |
| Tumor/lung | 7.62 | 70.57 | 62.72 | 105.33 | 1.82 |
| Tumor/liver | 6.93 | 12.59 | 7.9 | 5.96 | 0.19 |
| Tumor/muscle | 84.58 | 219.56 | 1129.0 | 105.33 | 10.0 |
| Tumor/skin | 8.50 | 56.46 | 161.29 | 26.33 | 6.67 |
2 h Data was cited from Table 1 for comparison.
p<0.05 for determining significance of differences in tumor and kidney uptake between 99mTc(CO)3-NOTA-GGNle-CycMSHhex with or without peptide blockade at 2 h post-injection.
Figure 4 illustrates the whole-body SPECT/CT images of B16/F10 melanoma-bearing mice at 2 h post injection of 99mTc(CO)3-NOTA-GGNle-CycMSHhex and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex, respectively. In agreement with the biodistribution results, the tumor lesions were clearly visualized by both peptides. However, as compared to 99mTc(CO)3-NODAGA-GGNle-CycMSHhex, 99mTc(CO)3-NOTA-GGNle-CycMSHhex exhibited higher melanoma uptake and lower renal uptake that were demonstrated in the biodistribution results in Table 1.
Figure 4.
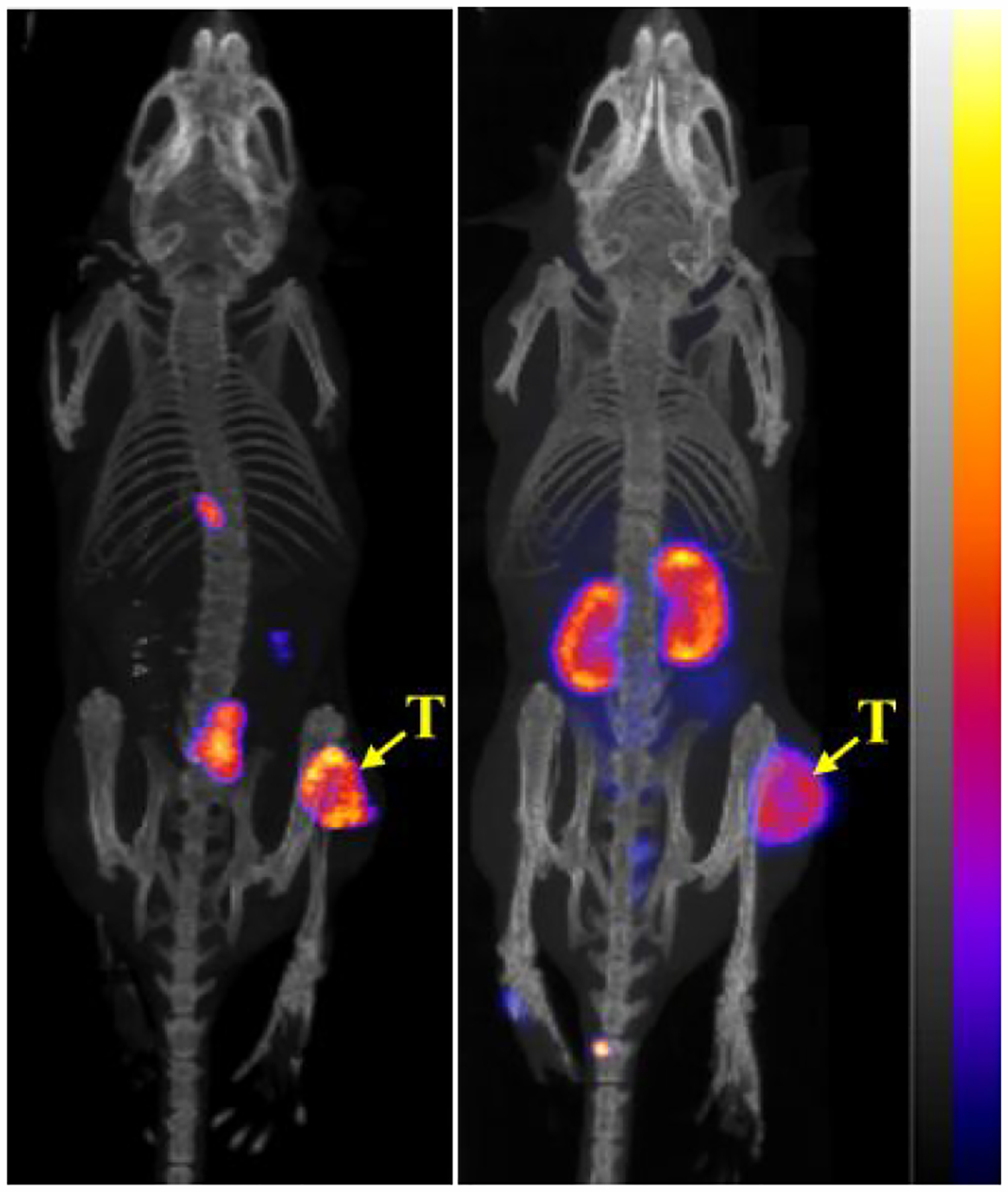
Representative maximum intensity projection SPECT/CT images of 99mTc(CO)3-NOTA-GGNle-CycMSHhex (left) and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex (right) on B16/F10 melanoma-bearing C57 mice at 2 h post-injection. The melanoma lesions (T) are highlighted with arrows.
DISCUSSION
99mTc is widely used in nuclear medicine due to its ideal decay properties, low-cost and high availability from commercial 99Mo/99mTc generator. Most 99mTcradiopharmaceuticals are readily prepared through the coordination of 99mTc=O3+ core (reduced from 99mTcO4−) by N and S atoms in bifunctional chelators attached to biomolecules. Subsequently, the development of [99mTc(CO)3(OH2)3]+ tricarbonyl kit offers another attractive and robust method for designing new 99mTcradiopharmaceuticals via replacing the labile water molecules with bifunctional chelators. Our previous study on 99mTc(CO)3-HYNIC-GGNle-CycMSHhex demonstrated the feasibility of HYNIC to coordinate 99mTc(CO)3 core. However, high liver and kidney uptake and moderate tumor uptake limited further evaluation of 99mTc(CO)3-HYNICGGNle-CycMSHhex. We previously reported that both 67Ga-NOTA-GGNle-CycMSHhex and 64Cu-NOTA-GGNle-CycMSHhex exhibited high melanoma uptake and contrast to normal organs, highlighting their potential for SPECT and PET imaging of melanoma.13, 14 Recent publication regarding high tumor uptake of 99mTc(CO)3-NOTA-sst2-ANT on AR42J tumor-bearing mice24 provided further impetus for us to explore the preparation of 99mTc(CO)3-NOTA/NODAGA-GGNle-CycMSHhex using [99mTc(CO)3(OH2)3]+ intermediate, and examine the tumor targeting and biodistribtution properties of 99mTc(CO)3-NOTA/NODAGA-GGNle-CycMSHhex in this study.
99mTc(CO)3-NOTA-GGNle-CycMSHhex displayed slightly higher MC1R-specific cellular uptake than 99mTc(CO)3-NODAGA-GGNle-CycMSHhex on B16/F10 cells. However, the tumor targeting and biodistribution properties were dramatically different between 99mTc(CO)3-NOTA-GGNle-CycMSHhex and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex. The B16/F10 melanoma uptake of 99mTc(CO)3-NOTA-GGNle-CycMSHhex was 19.76 ± 3.62% ID/g at 2 h post-injection which was 2.7 times the tumor uptake of 99mTc(CO)3-NODAGA-GGNle-CycMSHhex. Meanwhile, the renal uptake of 99mTc(CO)3-NOTA-GGNle-CycMSHhex was as low as 1.59 ± 0.52% ID/g at 2 h post-injection which was only 18% of that of 99mTc(CO)3-NODAGA-GGNle-CycMSHhex. High tumor uptake and low renal uptake clearly suggested 99mTc(CO)3-NOTA-GGNle-CycMSHhex as a lead peptide for further evaluation. Interestingly, as compared to our reported 99mTc(CO)3-HYNIC-GGNle-CycMSHhex,15 99mTc(CO)3-NODAGA-GGNle-CycMSHhex exhibited tremendous improvement in tumor, kidney and liver uptake. The tumor uptake of 99mTc(CO)3-NOTA-GGNle-CycMSHhex was 3.4 times the tumor uptake of 99mTc(CO)3-HYNIC-GGNle-CycMSHhex, whereas the renal and liver uptake of 99mTc(CO)3-NOTAGGNle-CycMSHhex was only 9% and 4% of that of 99mTc(CO)3-HYNIC-GGNle-CycMSHhex at 2 h post-injection,15 respectively. The tremendous differences in tumor targeting and biodistribution properties between 99mTc(CO)3-NOTA-GGNle-CycMSHhex and 99mTc(CO)3-HYNIC-GGNle-CycMSHhex were attributed to the difference in coordination chemistry of 99mTc(CO)3 between NOTA and HYNIC. The switch from HYNIC to NOTA dramatically increased the tumor uptake and decreased the renal and liver uptake of 99mTc(CO)3-NOTA-GGNle-CycMSHhex as compared to 99mTc(CO)3-HYNIC-GGNle-CycMSHhex.
99mTc(CO)3-NOTA-GGNle-CycMSHhex showed the highest tumor uptake at 2 h post-injection among different time points investigated, and high tumor to normal organ ratios after 2 h post-injection. The tumor uptake of 99mTc(CO)3-NOTA-GGNle-CycMSHhex was MC1R-specific, with 96% of the tumor uptake was blocked at 2 h post-injection. Meanwhile, the B16/F10 melanoma lesions could be clearly visualized by SPECT using 99mTc(CO)3-NOTA-GGNle-CycMSHhex as an imaging agent, demonstrating its potential use as an MC1R-targeting agent for melanoma detection. Moreover, 67Ga/64Cu-NOTAGGNle-CycMSHhex exhibited high melanoma uptake and potential for imaging of melanoma in our previous work.13, 14 Essentially, NOTA-GGNle-CycMSHhex could serve as a versatile peptide platform for radiolabeling of 99mTc, 67/68Ga3+ and 64Cu2+ radiometals for melanoma imaging. Depending upon the availability of radionuclides, generators, SPECT and PET machines, NOTA-GGNle-CycMSHhex could offer the flexibility to readily prepare the needed peptide radiopharmaceuticals for SPECT and PET imaging of melanoma. Meanwhile, the wide range of half-lives of 68Ga (68 min), 99mTc (6 h), 64Cu (12.7 h) and 67Ga (78.3 h) would also provide options for selection.
99mTc-(Arg11)CCMSH, 99mTc(CO)3-pz-βAla-Nle-cyclo[Asp-His-DPhe-Arg-Trp-Lys]-NH2 and 99mTc(EDDA)-HYNIC-GGNle-CycMSHhex were promising 99mTc-α-MSH peptides with high melanoma uptake reported in the literature.15, 25, 26 Among these peptides, 99mTc(EDDA)-HYNIC-GGNle-CycMSHhex showed the lowest renal uptake of 7.52 ± 0.96% ID/g at 2 h post-injection.15 Remarkably, the renal uptake of 99mTc(CO)3-NOTA-GGNle-CycMSHhex was only 21% of the renal uptake of 99mTc(EDDA)-HYNICGGNle-CycMSHhex, while the tumor uptake of 99mTc(CO)3-NOTA-GGNle-CycMSHhex was 40% more than that of 99mTc(EDDA)-HYNIC-GGNle-CycMSHhex at 2 h post-injection. Clearly, high melanoma uptake and low renal uptake of 99mTc(CO)3-NOTAGGNle-CycMSHhex warranted further investigation of 188Re(CO)3-NOTA-GGNle-CycMSHhex for melanoma therapy, taking advantage of similar coordination chemistry between matched-pair 99mTc/188Re theranostic radionuclides. The metal-cyclized 99mTc/188Re-(Arg11)CCMSH clearly demonstrated the matched-pair properties and promising theranostic results on B16/F1 murine and TXM13 human melanoma models.26–28 Interestingly, 99mTc(CO)3-NOTA-GGNle-CycMSHhex exhibited similar high tumor uptake as 99mTc-(Arg11)CCMSH but much lower renal uptake than 99mTc-(Arg11)CCMSH. According to the similar biodistribution results of 99mTc/188Re-(Arg11)CCMSH,26–28 it is reasonable to anticipate similar tumor targeting and biodistribution pattern for 188Re(CO)3-NOTA-GGNle-CycMSHhex. It is worthwhile to note that 188Re can be easily produced through a 188W/188Re generator which can be shipped to a pharmacy/research laboratory for on-site preparation. Therefore, it would be interesting to prepare and evaluate 188Re(CO)3-NOTA-GGNle-CycMSHhex for melanoma therapy in future studies.
CONCLUSIONS
The melanoma targeting and imaging properties of 99mTc(CO)3-NOTA-GGNle-CycMSHhex and 99mTc(CO)3-NODAGA-GGNle-CycMSHhex were determined on B16/F10 melanoma bearing C57 mice. 99mTc(CO)3-NOTA-GGNle-CycMSHhex exhibited higher tumor uptake and lower renal uptake than 99mTc(CO)3-NODAGA-GGNle-CycMSHhex at 2 h post-injection. High tumor uptake, low kidney uptake and fast urinary clearance of 99mTc(CO)3-NOTA-GGNle-CycMSHhex highlighted its potential for melanoma imaging and warranted the evaluation of 188Re(CO)3-NOTA-GGNle-CycMSHhex for melanoma therapy.
ACKNOWLEDGMENTS
We thank Dr. Fabio Gallazzi for his technical assistance. This work was partially supported by NIH grant R01CA225837 and University of Colorado Denver startup fund.
REFERENCES
- 1.Siegel RL; Miller KD; Jemal A Cancer statistics, 2020. CA Cancer J. Clin 2020, 70, 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Chapman PB; Hauschild A; Robert C; et al. BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med 2011, 364, 2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sosman JA; Kim KB; Schuchter L; et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N. Engl. J. Med 2012, 366, 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodi FS; O’Day SJ; McDermott DF; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med 2010, 363, 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber JS; O’Day SJ; Urba W; et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J. Clin. Oncol 2008, 26, 5950–5956. [DOI] [PubMed] [Google Scholar]
- 6.Topalian SL; Sznol M; McDermott DF; et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol 2014, 32, 1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss SA; Wolchok JD; Sznol M Immunotherapy of melanoma: facts and hopes. Clin. Cancer Res 2019, 25, 5191–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siegrist W; Solca F; Stutz S; et al. Characterization of receptors for alpha-melanocyte-stimulating hormone on human melanoma cells. Cancer Res. 1989, 49, 6352–6358. [PubMed] [Google Scholar]
- 9.Tatro JB; Wen Z; Entwistle ML; et al. Interaction on an α-melanocyte stimulating hormone-diptheria toxin fusion protein with melanotropin receptors in human metastases. Cancer Res. 1992, 52, 2545–2548. [PubMed] [Google Scholar]
- 10.Yang J; Xu J; Gonzalez R; Lindner T; Kratochwil C; Miao Y 68Ga-DOTAGGNle-CycMSHhex targets the melanocortin-1 receptor for melamoma imaging. Sci. Transl. Med 2018, 10, eaau4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo H; Yang J; Gallazzi F; Miao Y Reduction of the ring size of radiolabeled lactam bridge-cyclized alpha-MSH peptide resulting in enhanced melanoma uptake. J. Nucl. Med 2010, 51, 418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo H; Yang J; Gallazzi F; Miao Y Effects of the amino acid linkers on melanoma-targeting and pharmacokinetic properties of Indium-111-labeled lactam bridge-cyclized α-MSH peptides. J. Nucl. Med 2011, 52, 608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo H; Gallazzi F; Miao Y Ga-67-labeled lactam bridge-cyclized alpha-MSH peptides with enhanced melanoma uptake and reduced renal uptake. Bioconjug. Chem 2012, 23, 1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo H; Miao Y Cu-64-labeled lactam bridge-cyclized alpha-MSH peptides for PET imaging of melanoma. Mol. Pharm 2012, 9, 2322–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo H; Gallazzi F; Miao Y Design and evaluation of new Tc-99m-labeled lactam bridge-cyclized alpha-MSH peptides for melanoma imaging. Mol. Pharm 2013, 10, 1400–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo H; Miao Y Introduction of an aminooctanoic acid linker enhances uptake of Tc-99m-labeled lactam bridge-cyclized alpha-MSH peptide in melanoma. J. Nucl. Med 2014, 55, 2057–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo H; Miao Y Melanoma targeting property of a Lu-177-labeled lactam bridge-cyclized alpha-MSH peptide. Bioorg. Med. Chem. Lett 2013, 23, 2319–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J; Xu J; Cheuy L; Gonzalez R; Fisher DR; Miao Y Evaluation of a novel Pb-203-labeled lactam-cyclized alpha-melanocyte-stimulating hormone peptide for melanoma targeting. Mol. Pharm 2019, 16, 1694–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J; Yang J; Gonzalez R; Fisher DR; Miao Y Melanoma-targeting property of Y-90-labeled lactam-cyclized alpha-melanocyte-stimulating hormone peptide. Cancer Biother. Radiopharm 2019, 34, 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki K; Shimmura N; Thipyapong K; Uehara T; Akizawa H; Arano Y Assessment of macrocyclic triamine ligands as synthons for organometallic 99mTcradiopharmaceuticals. Inorg. Chem 2008, 47, 2593–2600. [DOI] [PubMed] [Google Scholar]
- 21.Braband H; Imstepf S; Benz M; Spingler B; Alberto R Combining bifunctional chelator with (3+2)-cycloaddition approaches: synthesis of dual-function technetium complexes. Inorg. Chem 2012, 51,4051–4057. [DOI] [PubMed] [Google Scholar]
- 22.Veerendra B; Sieckman G; Hoffman T; et al. Synthesis, radiolabeling and in vitro GRP receptor targeting studies of 99mTc-triaza-X-BBN[7−14]NH2 (X = Serylserylserine, Glycylglycylglycine, Glycylserylglycine, or Beta Alanine). Synth. React. Inorg. Met-Org., Nano-Met Chem 2006, 36, 481–491. [Google Scholar]
- 23.Makris G; Radford LL; Kuchuk M; et al. NOTA and NODAGA [99mTc]Tc- and [186Re]Re-tricarbonyl complexes: radiochemistry and first example of a [99mTc]Tc-NODAGA somatostatin receptor-targeting bioconjugate. Bioconjug. Chem 2018, 29, 4040–4049. [DOI] [PubMed] [Google Scholar]
- 24.George M; Kuchuk M; Gallazzi F; Jurisson SS; Smith CJ; Hennkens HM Somatostatin receptor targeting with hydrophilic [99mTc/186Re]Tc/Re-tricarbonyl NODAGA and NOTA complexes. Nucl. Med. Biol 2019, 71, 39–46. [DOI] [PubMed] [Google Scholar]
- 25.Raposinho PD; Correia JD; Alves S; Botelho MF; Santos AC; Santos IA 99mTc(CO)3-labeled pyrazolyl-α-melanocyte-stimulating hormone analog conjugate for melanoma targeting. Nucl. Med. Biol 2008, 35, 91–99. [DOI] [PubMed] [Google Scholar]
- 26.Miao Y; Benwell K; Quinn TP 99mTc and 111In labeled alpha-melanocyte stimulating hormone peptides as imaging probes for primary and pulmonary metastatic melanoma detection. J. Nucl. Med 2007, 48, 73–80. [PubMed] [Google Scholar]
- 27.Miao Y; Owen NK; Whitener D; Gallazzi F; Hoffman TJ; Quinn TP In vivo evaluation of 188Re-labeled alpha-melanocyte stimulating hormone peptide analogs for melanoma therapy. Int. J. Cancer 2002, 101, 480–487. [DOI] [PubMed] [Google Scholar]
- 28.Miao Y; Owen NK; Fisher DR; Hoffman TJ; Quinn TP Therapeutic efficacy of a 188Re-labeled alpha-melanocyte-stimulating hormone peptide analog in murine and human melanoma-bearing mouse models. J. Nucl. Med 2005, 46, 121–129. [PubMed] [Google Scholar]


