The authors of the original article “Unpuzzling COVID-19: tissue-related signaling pathways associated with SARS-CoV-2 infection and transmission” (Clin Sci (Lond) (2020) 134(16), DOI: 10.1042/CS20200904) would like to correct Figures 2 and 3 of their article as they have noted that these were inverted at article submission. The figures are presented with their correct order here.
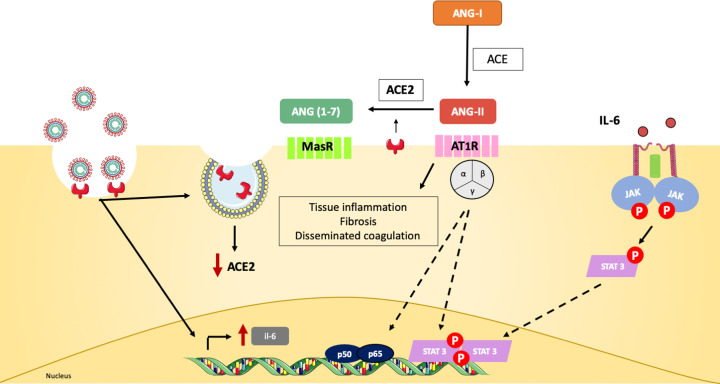
Figure 2. Canonical ACE2 pathway links multiple organ damage in COVID-19.
SARS-CoV-2 infection down-regulates ACE2 expression and leads to the production of pro-inflammatory mediators, such as IL-6 [1]. Angiotensin-I (Ang-I) is converted into Ang-II by the ACE in the extracellular space. ACE2 is able to further cleave Ang-II to Ang(1-7), which binds MasR receptors on the cell surface and promotes anti-inflammatory, vasodilation and anti-fibrotic effects [1]. Since ACE2 is down-regulated during viral infection, this event will lead to the accumulation of Ang-II and binding to AT1R receptors on cellular membrane. AT1R signals through JAK-STAT and induces fibrosis, pro-inflammatory gene expression and vasoconstriction [2,3]. Multiple organs express ACE2 and are target for SARS-CoV-2. As they lose ACE2-mediated protection, Ang-II signaling contributes to the pathological findings observed in COVID-19 patients, such as disseminated coagulopathy and acute tissue damage [4].
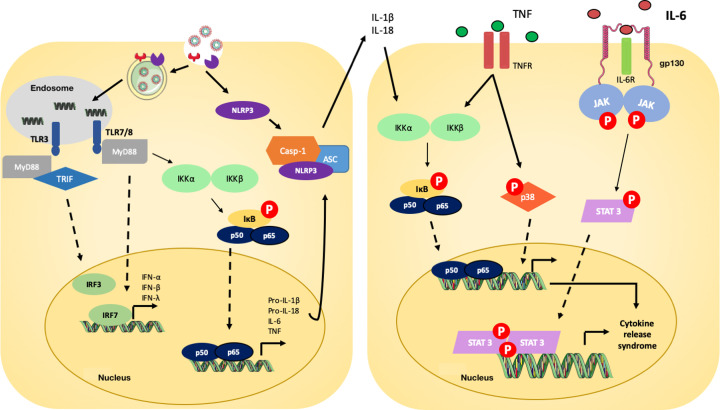
Figure 3. Signaling pathways involved in COVID-19 pathophysiology.
Toll-like receptors (TLRs) 3 and TLR 7/8 recognize SARS-CoV-2 RNA and initiate the inflammatory cascade via type I and type II IFN gene expression and NF-κB nuclear translocation [5,6]. Via NF-κB, the expression of multiple pro-inflammatory genes is stimulated, including pro-IL-1β, pro-IL-18, TNF and IL-6 [7–9]. The virus is also recognized by cytoplasmic NLRP3, which forms, together with ASC and caspase-1 (Casp-1), the inflammasome complex that will cleave and release mature forms of IL-1β and IL-18 [10]. The cytokines IL-1β, IL-18 and TNF bind to specific receptors and promote further NF-κB nuclear translocation and phosphorylation of p38 MAPK, which will lead to great expression of pro-inflammatory cytokines and chemokines [11,12]. IL-6, an important player in COVID-19, binds IL-6R and gp130 receptors to activate JAK/STAT-3 pathway and then contribute to the CRS observed in COVID-19 patients [13].
The authors apologise for any inconvenience caused to the reader.
References
- 1.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.-C., Turner A.J. et al. (2020) Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 126, 1456–1474 10.1161/CIRCRESAHA.120.317015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perrotta F., Matera M.G., Cazzola M. and Bianco A. (2020) Severe respiratory SARS-CoV2 infection: Does ACE2 receptor matter? Respir. Med. 168, 105996 10.1016/j.rmed.2020.105996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groß S., Jahn C., Cushman S., Bär C. and Thum T. (2020) SARS-CoV-2 receptor ACE2-dependent implications on the cardiovascular system: From basic science to clinical implications. J. Mol. Cell Cardiol. 144, 47–53 10.1016/j.yjmcc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian S., Xiong Y., Liu H., Niu L., Guo J., Liao M. et al. (2020) Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod. Pathol. 10–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabroe I., Parker L.C., Dower S.K. and Whyte M.K.B. (2008) The role of TLR activation in inflammation. J. Patho. 214, 126–135 10.1002/path.2264 [DOI] [PubMed] [Google Scholar]
- 6.Totura A.L., Whitmore A., Agnihothram S., Schäfer A., Katze M.G., Heise M.T. et al. (2015) Toll-like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. MBio 6, 1–14 10.1128/mBio.00638-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q. and Verma I.M. (2002) NF-κB regulation in the immune system. Nat. Rev. Immunol. 2, 725–734 10.1038/nri910 [DOI] [PubMed] [Google Scholar]
- 8.Tergaonkar V., Correa R.G., Ikawa M. and Verma I.M. (2005) Distinct roles of IκB proteins in regulating constitutive NF-κB activity. Nat. Cell Biol. 7, 921–923 10.1038/ncb1296 [DOI] [PubMed] [Google Scholar]
- 9.Tergaonkar V. (2006) NFκB pathway: A good signaling paradigm and therapeutic target. Int. J. Biochem. Cell Biol. 38, 1647–1653, Available from: https://linkinghub.elsevier.com/retrieve/pii/S1357272506001245 10.1016/j.biocel.2006.03.023 [DOI] [PubMed] [Google Scholar]
- 10.Zhao C. and Zhao W.. NLRP3 Inflammasome — A Key Player in Antiviral Responses 2020111–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimes J.M. and Grimes K.V. (2020) p38 MAPK inhibition: A promising therapeutic approach for COVID-19. J. Mol. Cell Cardiol. 144, 63–65 10.1016/j.yjmcc.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Y., Fang Z., Liu B. and Zheng X. (2019) P38MAPK plays a pivotal role in the development of acute respiratory distress syndrome. Clinics 74, 1–5 10.6061/clinics/2019/e509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C., Wu Z., Li J.W., Zhao H. and Wang G.Q. (2020) The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents 55, 10.1016/j.ijantimicag.2020.105954 [DOI] [PMC free article] [PubMed] [Google Scholar]