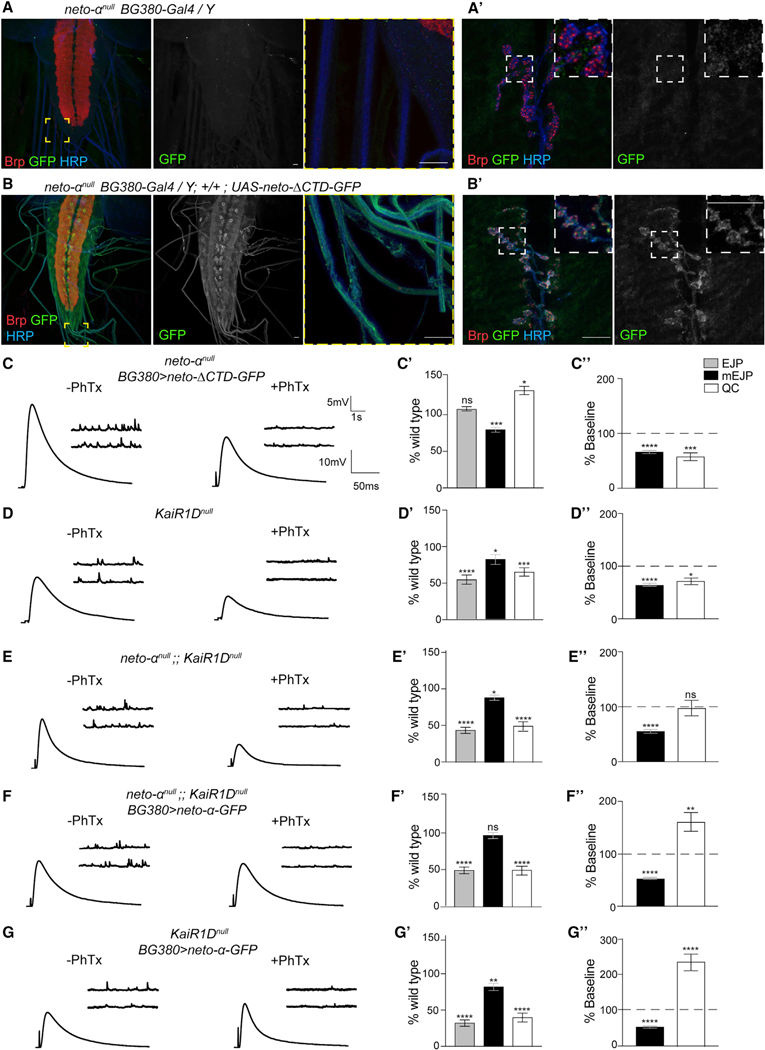
Figure 7. Distinct Domains of Neto-α Regulate Basal Release and Presynaptic Potentiation.
(A–B’) Confocal images of the ventral ganglia (A and B) and NMJ boutons (A’ and B’) labeled for Brp (red), GFP (green), and HRP (blue) showing the distribution of Neto-ΔCTD-GFP when overexpressed in MNs (A) and the negative control (B). Similar to Neto-α-GFP (Figure 5C), Neto-ΔCTD-GFP labels the soma and axons of MNs and accumulates at synaptic terminals.
(C–G’) Sets of electrophysiological recordings of basal neurotransmission and presynaptic homeostatic potentiation response for the indicated genotypes. Each analysis includes representative traces for mEJP and EJP recordings before and after PhTx application (left); quantification of mEJP amplitude, EJP amplitude, and QC values normalized to control (w1118) (middle); and quantification of mEJP amplitude and QC relative values after PhTx treatment normalized to the baseline values of the same genotype (right). Neuronal Neto-ΔCTD-GFP rescues basal neurotransmission but cannot restore the homeostatic response at neto-anull NMJs (C). The electrophysiological defects of KaiRIDnull NMJs resemble those of neto-αnull mutants, as well as of neto-αnull;;KaiRIDnull, suggesting that KaiRID and Neto-α function in the same pathway (D and E) (Figure S5). Overexpression of neto-α-GFP in neto-αnull;;KaiRIDnull MNs does not restore the EJP amplitude but does enable a significant PHP response (F). When neto-α-GFP is overexpressed in the presence of endogenous Neto-α, the amplitude of the PHP response is dramatically increased (G).
Scale bars: 10 μm. Data are represented as mean ± SEM. ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05; ns, p > 0.05.