Abstract
COVID-19 (Coronavirus disease 2019) is a transmissible disease initiated and propagated through a new virus strain SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus-2) since 31st December 2019 in Wuhan city of China and the infection has outspread globally influencing millions of people. Here, an attempt was made to recognize natural phytochemicals from medicinal plants, in order to reutilize them against COVID-19 by the virtue of molecular docking and molecular dynamics (MD) simulation study. Molecular docking study showed six probable inhibitors against SARS-CoV-2 Mpro (Main protease), two from Withania somnifera (Ashwagandha) (Withanoside V [10.32 kcal/mol] and Somniferine [9.62 kcal/mol]), one from Tinospora cordifolia (Giloy) (Tinocordiside [8.10 kcal/mol]) and three from Ocimum sanctum (Tulsi) (Vicenin [8.97 kcal/mol], Isorientin 4′-O-glucoside 2″-O-p-hydroxybenzoagte [8.55 kcal/mol] and Ursolic acid [8.52 kcal/mol]). ADMET profile prediction showed that the best docked phytochemicals from present work were safe and possesses drug-like properties. Further MD simulation study was performed to assess the constancy of docked complexes and found stable. Hence from present study it could be suggested that active phytochemicals from medicinal plants could potentially inhibit Mpro of SARS-CoV-2 and further equip the management strategy against COVID-19-a global contagion.
Highlights
Holistic approach of Ayurvedic medicinal plants to avenge against COVID-19 pandemic.
Active phytoconstituents of Ayurvedic medicinal plants Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi) predicted to significantly hinder main protease (Mpro or 3Clpro) of SARS-CoV-2.
Through molecular docking and molecular dynamic simulation study, Withanoside V, Somniferine, Tinocordiside, Vicenin, Ursolic acid and Isorientin 4′-O-glucoside 2″-O-p-hydroxybenzoagte were anticipated to impede the activity of SARS-CoV-2 Mpro.
Drug-likeness and ADMET profile prediction of best docked compounds from present study were predicted to be safe, drug-like compounds with no toxicity.
Communicated by Ramaswamy H. Sarma
Keywords: COVID-19 (SARS-CoV-2) Mpro, molecular docking, MD simulation, ayurveda, medicinal plants, ADMET, drug-likeness
Graphical Abstract
1. Introduction
As we pen down this sentence, cases of COVID-19 (Coronavirus disease 19) are continuously increasing across the globe. In accordance to 26th July 2020 as per WHO (World Health Organization) situation report No. 187 and Centre for System Science and Engineering, Johns Hopkins University, globally greater than 15.9 million cases have been stated, resulting in 643,000 deaths, 9.22 million recovered cases with United States reporting the highest number of cases around 4.17 million (World Health Organization (WHO), 2020) (Johns Hopkins CSSE, 2020). Speaking for India as per Ministry of Health and Family Welfare, till 26th July 2020 there are 1,385,522 confirmed cases, and 467,882 active cases, among which 32,063 deaths and 885,576 recovered cases have been reported (Policy | Ministry of Health and Family Welfare | GOI, Policy | Ministry of Health and Family Welfare | GOI, 2020Policy | Policy | Ministry of Health & Family Welfare | GOI, 2020Welfare | GOI, 2020). Coronaviruses (CoV) is the beta strain from the family Coronaviridae which leads to maladies of the vital organs like respiratory, enteric, hepatic and neurological systems if not treated (Chan et al., 2012; Zumla et al., 2016). It is a single stranded RNA virus (positive sense) having diameter of 80–120 nm, among which recently SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus-2) has arose as a universal contagion infecting humans with 3–4% mortality rate (Zhu et al., 2020). Owing to zoonotic origins and genetic similarity, bats are pondered as the natural hosts of SARS-CoV-2 (Ye et al., 2020). It consists of four structural proteins namely Spike (S), Envelope (E), Membrane (M) and Nucleocaspid (N) which helps coronavirus in recognizing the receptor on the target cell, fusion with membrane receptor causing infection and further transmission within the host. As compared to SARS-CoV, novel SARS-CoV-2 binds ACE2 (Angiotensin Converting Enzyme-2) host surface protein with affinity far greater than threshold requisite for virus infectivity, but the pathological effects of SARS-CoV-2 for causing organ damage remained to be elucidated (Wang et al., 2020). This binding factor is the major reason for swift transmission potentiality of the SARS-CoV-2 as compared to SARS-CoV (Walls et al., 2020). The first line of action is to hinder the latching of virus towards the host ACE-2 receptors by blocking the function of Spike proteins, which would help to trim down the figure of new cases. But in already infected case to provide remedy, target for main proteases (Mpro) are being searched out as it translates the viral RNA into functional polyproteins that alters the normal physiology of the subject by formation of viral RNA polymerase, endoribonuclease and exoribonuclease (Khan et al., 2020; Elmezayen et al., 2020). Mpro, also called 3CLpro (3C-like protease or chymotrypsin-like protease) is a dimer owning six stranded antiparallel β barrels, holding substrate binding site in the middle of them, along with cluster of five helices which are responsible for dimerization of the enzyme. The majority of the Coronaviridae genome encodes two polyproteins, pp1a (Wrapp et al., 2020) and pp1ab (Mengist et al., 2020; Mittal et al., 2020). With the help of two proteases 3CLpro (3-chemotrypsin-like protease) and PLpro (papain-like protease) encoded by the open reading frame, the above mentioned polyproteins are cleaved and further transformed in mature non-structural proteins (NSPs). In addition to papain-like protease(s) Mpro is required for dispensing polyproteins at 11 different sites to produce proteins which are translated from the viral RNA. Restraining the pursuit of Mpro would impede viral replication process inside the host (Anand et al., 2003; Zhang et al., 2020). Since no other human protease are known with similar cleavage specificity at sites of Leu-Gln↓(Ser, Ala, Gly) is known till date, the inhibitors of these targets are likely to be non-toxic. Amongst the prospective protease inhibitors, the antivirals Nelfinavir, Remdesivir, Lopinavir and Ritonavir along with α-ketoamide are peculiarly striking as therapeutics to battle the new coronavirus (De Oliveira et al., 2020; Mothay & Ramesh, 2020). Apart from these, combination of anti-viral such as Lopinavir, Ritonavir, Favipiravir (Khan et al., 2020), anti-malarial, namely Chloroquine, Hydroxychloroquine (Muralidharan et al., 2020) and corticosteroid like Dexamethasone (World Health Organization, 2020) therapy are being used for controlling COVID-19. Therefore, SARS-CoV-2 Mpro have recently emerged as the better targets in inhibiting virus replication. The present study fascinated on the main proteases of SARS-CoV-2 as potential macromolecular target for COVID-19 management using active phytoconstituents mentioned in Ayurvedic scriptures.
Ayurveda knows as ‘The Science of Life’, an ancient system of medicine practiced in Indian subcontinent; based on the holistic principle of life, health, and healing system. A group of rejuvenative methods that imparts biological nourishment to the body tissues was described in Ayurvedic text (Singh et al., 2008). It describe many medicinal plants with wide range of therapeutic potentiality in treating the ailment of respiratory system; some of the notable ones but not limited to are Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy), Ocimum sanctum (Tulsi) used in present study. They are all immunomodulators and strengthen the body against infections. W. somnifera (WS), usually known as Ashwagandha, categorized as a rasayana (rejuvenator) which was predicted to enrich physical and mental state, considered to rejuvenate the body in weakened situations and upturns longevity (Williamson, 2002). It has been reported to have anti-inflammatory, anti-diabetic, antimicrobial, analgesic, anti-tumour, anti-stress, neuroprotective, cardioprotective, rejuvenating and immunomodulatory effects (Kapoor, 1990; Ven Murthy et al., 2010; Vyas et al., 2011; Williamson, 2002). An active ingredient known as ‘withanolides’ contains steroidal saponin, alkaloids, and steroidal lactones. Most of biological action contributed by Withaferin-A and Withanolide D, along with Withanoside I–VII, new Withanolide Glycosides, extracted from its roots (Matsuda et al., 2001). T. cordifolia (Guduchi or Giloy), is a medicinal plant which has been used for its remedial purpose for thousands of year in Ayurvedic system of medicine. Its extracts have alkaloids, glycosides, steroids and polysaccharides (Panchabhai et al., 2008). It is well known for its immunomodulatory, antidiabetic, antioxidant, antihepatotoxic and cytotoxic effects (Sharma et al., 2012; Thatte et al., 1992). The active phytoconstituents, Tinocordioside, Cordifolioside A, Magnoflorine, and Syringin are known for its immunomodulatory effect (Sharma et al., 2011). O. sanctum (Tulsi), as a sanctified herb used for its medicinal property as per Ayurvedic scriptures. It’s multiple therapeutic action comprises of adaptogenic, immunomodulatory, antimicrobial, cardioprotective, and anti-inflammatory effects (Jamshidi & Cohen, 2017), anti‑viral, anti‑fungal and anti‑bacterial activity (S. Sharma, 1999), also possess anti-diabetic, analgesic, antifertility, anticancer, antispasmodic, antiemetic, diaphoretic and hepatoprotective actions (Prakash & Gupta, 2005). Their leaves are beneficial for the treatment of rheumatism, bronchitis, and pyrexia and considered as ‘Elixir of life’ for its healing power (Nadkarni & Krishnarao, 1954). By the enhancement of both cellular and humoral immunity, it strengthens the immune response (Mukherjee et al., 2005). Eugenol and Ursolic acid are its main phytoconstituents (Prakash & Gupta, 2005).
According to previous studies it has been reported that some of the phytoconstituents from above medicinal plants shows interaction with SARS CoV- 2 Mpro, and other target proteins (S, E, N) of COVID-19, these phytoconstituents are Withaferin A, Withanolide B, Tinocordioside, Somniferine A, Tinosporide, Withanolide, Orientin, Flavonol glucoside, Apigenin, Kaempferol, Withanone (Dharmendra & Deepak, 2020). It has been reported that Dihydrodieuginol B and Tulsinol A, B, C, D, E, F, G of O. sanctum could be used as potential inhibitors for Papain-like Protease and SARS Coronavirus Main Protease (Varshney et al., 2020) and Withanone from W. somnifera could be used to disrupt interconnections between Viral S-Protein RBD (receptor binding domain) and ACE2 host receptor of COVID-19 (Balkrishna et al., 2020). With majority of world beneath lockdown and the alarming menace of millions of deaths, researchers requisite to find an effective drug more rapidly. Since SARS-CoV-2, is a new virus, more studies are required for searching the drugs to target viral systems without much side-effect to the human populations; here Ayurvedic formulations can play a significant role, for developing therapeutic moieties that would be safe and nontoxic. In this research article, with the use of in silico molecular docking and molecular dynamic simulation studies, an attempt was made to identify new active and stable inhibitors against novel coronavirus main protease, from total 102 different active phytoconstituents from above stated medicinal plants. Thereby hindering the viral translation into functional polyproteins required for further replication of virus and disruption of host physiological/biological functions.
2. Materials and methods
2.1. Protein preparation
RCSB (Research Collaboratory for Structural Bioinformatics) Protein Data Bank (https://www.rcsb.org/) was used to retrieve the three dimensional crystal structure of COVID-19 (SARS-CoV-2) Mpro (PDB ID-6LU7) with crystal resolution of 2.16 Å. It has only one chain of total 306 amino acids. Protein preparation was done with the help of ‘Prepare protein’ protocol of Discovery studio 4.0 (DS 4.0). Heteroatoms and water molecules present in the crystal structure were removed at physiological pH 7.4 of DS 4.0. Further, the active site prediction of the prepared protein was done by DS 4.0.
2.2. Ligands selection
For the documentation of potential inhibitors of SARS-CoV-2 Mpro, total 102 active phytochemicals comprising of 28 from medicinal plants W. somnifera (Ashwagandha) (Dhar et al., 2006; Ganguly et al., 2018) (Table S1), 28 from T. cordifolia (Giloy) (Chaudhary et al., 2020) (Sharma et al., 2019) (Table S2), and 46 from O. sanctum (Tulsi) (Chaudhary et al., 2020) (Table S3) were retrieved from literature. PubChem compound database (https://pubchem.ncbi.nlm.nih.gov/) was used for the retrieval of structures in 2 D SDF (Two Dimensional Structure Data File) format. Afterwards ligand optimization, energy minimization and conversion of retrieved ligands to 3 D PDB format were done with the help of DS 4.0.
Table 1.
List of phytochemicals with binding energy >8.0 kcal/mol for SARS-CoV-2 Mpro.
| Compounds | Target molecule; Binding energy (kcal/mol) (>8.0 kcal/mol) |
|---|---|
| SARS-CoV-2 Mpro (PDB ID:6LU7) | |
| Native ligand (N3) | 8.52 |
| Phytochemicals | |
| Withanoside V | 10.32 |
| Somniferine | 9.62 |
| Tinocordiside | 8.10 |
| Vicenin | 8.97 |
| Isorientin 4′-O-glucoside 2″-O-p-hydroxybenzoagte | 8.55 |
| Ursolic acid | 8.52 |
Table 2.
Physiochemical properties of potential inhibitors from Ayurvedic medicinal plants.
| Compound name | MW < 500 | HD < 5 | HA < 10 | Log p < 5 | MR (40–130) | HIA | Log S>-5 | Caco-2 (cm/s) | Carcinogens |
|---|---|---|---|---|---|---|---|---|---|
| Withanoside V | 766 | 1 | 14 | 2.88 | 182.17 | 0.7051 | −4.2128 | 0.9403 | NC |
| Somniferine | 608 | 2 | 9 | 2.71 | 161.30 | 0.9966 | −2.4908 | 0.6432 | NC |
| Tinocordiside | 396 | 4 | 7 | 0.38 | 98.99 | 0.6702 | −3.2148 | 0.8143 | NC |
| Vicenin | 594 | 11 | 15 | −2.55 | 136.25 | 0.9156 | −2.1712 | 0.9096 | NC |
| Isorientin 4′-O-glucoside 2″-O-p-hydroxybenzoagte | 730 | 11 | 18 | −1.31 | 169.23 | 0.5493 | −2.3863 | 0.9338 | NC |
| Ursolic acid | 456 | 2 | 3 | 7.08 | 132.61 | 1.0000 | −4.3883 | 0.8353 | NC |
Note: NC, Non-carcinogenic.
2.3. Molecular docking
AutoDock Vina-based YASARA (Yet Another Scientific Artificial Reality Application) software was used for molecular docking study (Krieger et al., 2014)(Trott & Olson, 2009). Using YASARA, selected 28, 28 and 46 (Total 102) active phytochemicals of W. somnifera (Ashwagandha), T. cordifolia (Giloy) and O. sanctum (Tulsi) were docked with COVID-19 (SARS-CoV-2) Mpro (PDB ID: 6LU7). For docking study prepared receptor and ligand files were used for setting target and play macro in YASARA software. The macro file dockrun_mcr was used for the calculation of interaction energy among receptor and selected ligands independently. 25 VINA docking runs of the ligand object 2 to the receptor object 1 was done with the help of YASARA. Afterwards, with the help of YASARA software, docked complexes were visualized and converted in PDB files for 2 D-3D interactive visualization study with the help of DS 4.0 and PyMol software (Yuan et al., 2017). For the docking calculation study, the result log files obtained from YASARA were taken. Shortening of docked complexes were done based on binding energy (kcal/mol) and dissociation constant (pM) as per YASARA scoring where positive energy means stronger binding and negative energy means no binding.
2.4. Molecular dynamics simulation
Protein preparation wizard of OPLS-3e (optimized potential for liquid simulations-Schrodinger) force field was used for preparation, optimization and minimization of the docked complexes. The least energy minimized complexes with the 0.30 Å RMSD (root mean square deviation) were exposed to molecular dynamics (MD) simulations with the help of Desmond MD package (Bowers et al., 2006; Chow et al., 2008). Initial system was built to cover the protein ligand complexes with the TIP3P water model using a system builder. For neutralization, appropriate number of metal ions was added with the salt concentration of 0.15. Build-ed systems of each complex were subjected to standard equilibration protocol (Selvaraj et al., 2018). MD simulation was performed for the timescale of 20 nano seconds (ns) using OPLS-3e force field. The results of RMSD and hydrogen bonds were analysed using the Desmond simulation interaction analysis (Yadav et al., 2020).
2.5. Drug-likeness and ADMET prediction
The best docked compounds from W. somnifera (Ashwagandha), T. cordifolia (Giloy), and O. sanctum (Tulsi) were taken for drug-likeness test and ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicity) profile prediction with the help of web based server Lipinski rule of five (http://www.scfbio-iitd.res.in/software/drugdesign/lipinski.jsp) (Jayaram et al., 2012; Lipinski, 2004) and admetSAR server (http://lmmd.ecust.edu.cn/admetsar1/predict/) (Cheng et al., 2012).
3. Results
Molecular docking
As per YASARA scoring, molecular docking study revealed that different active phytochemicals present in W. somnifera (Ashwagandha), T. cordifolia (Giloy) and O. sanctum (Tulsi) exhibited significant binding affinity with SARS-CoV-2 Mpro. Table 1 represents the list of phytochemicals displaying significant binding energy (>8.0 kcal/mol) with SARS-CoV-2 Mpro.
Potential inhibitors from W. somnifera (Ashwagandha) for SARS-CoV-2 Mpro
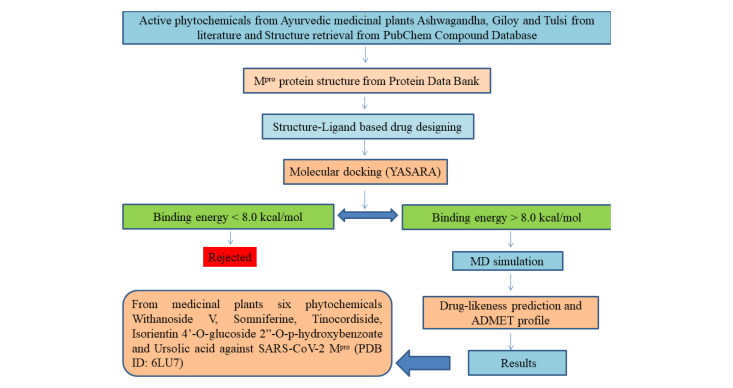
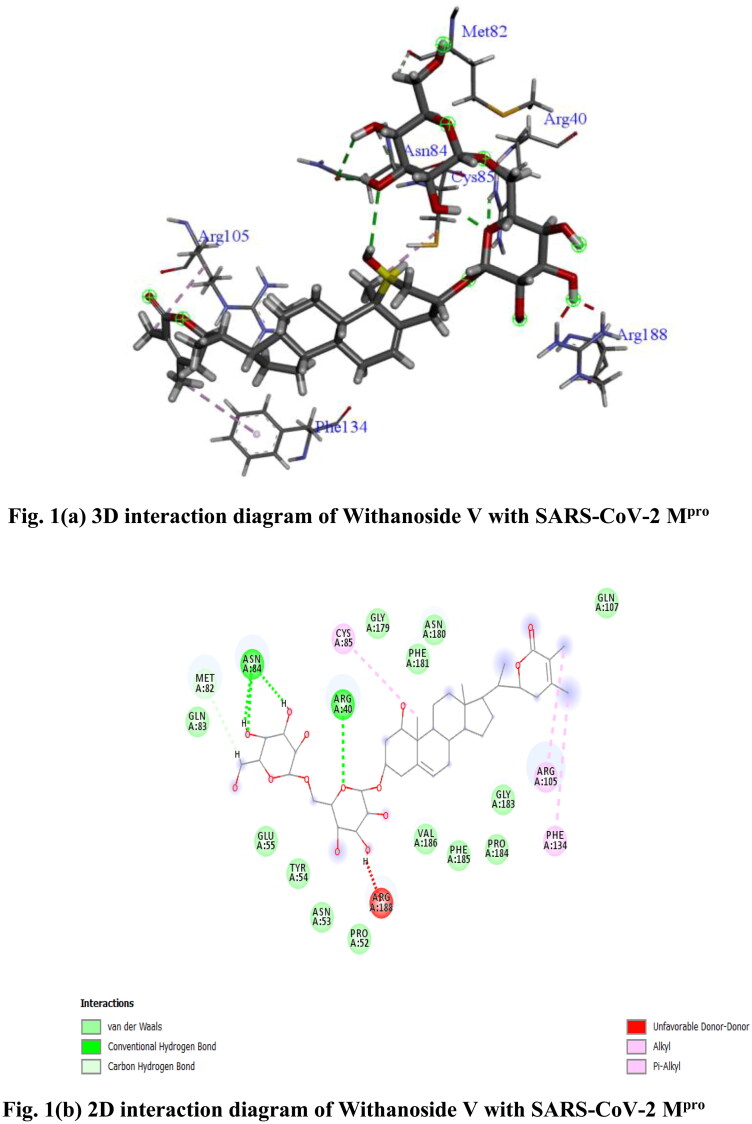
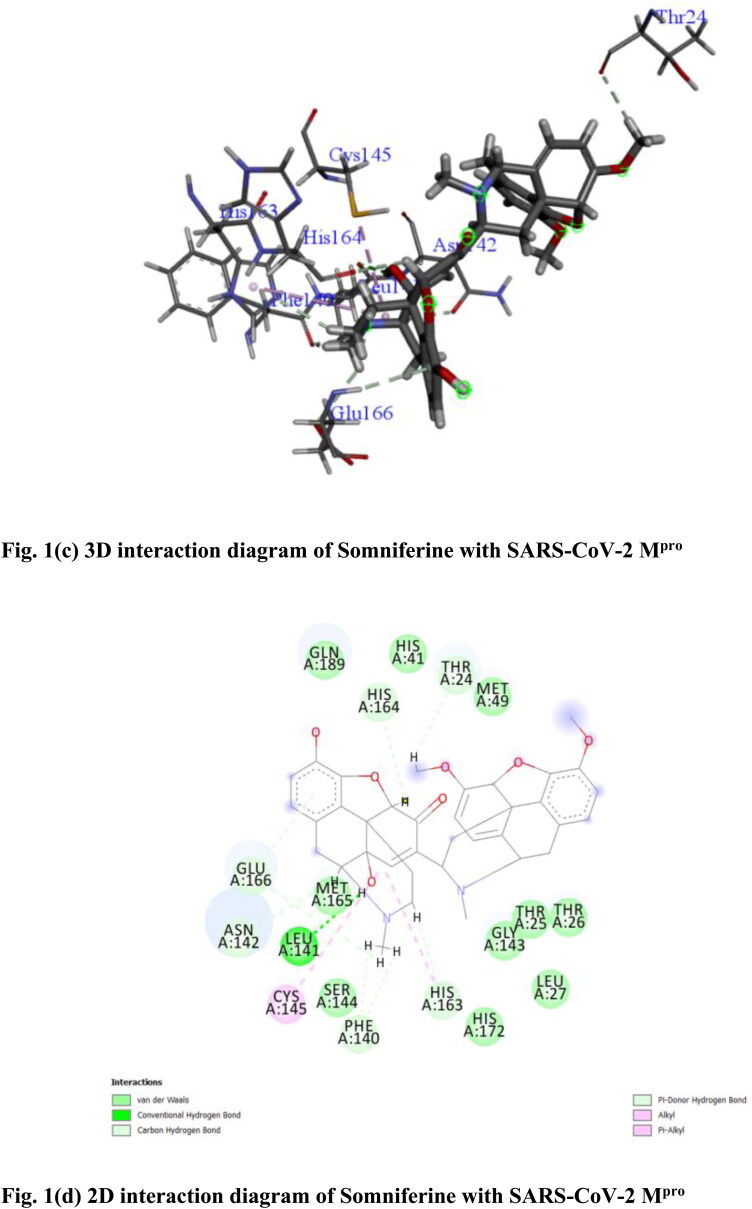
From molecular docking study it has been found that out of 28 compounds from W. somnifera (Ashwagandha) only two compounds namely Withanoside V (CID_10700345) and Somniferine (CID_14106343) showed significant binding affinity as compared to native N3 (CID_146025593) for SARS-CoV-2 Mpro as per YASARA scoring. Withanoside V showed highest binding energy of 10.32 kcal/mol. Withanoside V is a glycoside obtained from W. somnifera roots, reported to have tachyphylaxis inhibition activity (Matsuda et al., 2001). It forms different ligand–protein 2D–3D interactions which includes conventional and carbon hydrogen bonding with residue Asn 84, Arg 40 and Met 82, alkyl and π-alkyl interaction with Cys 85, Arg 105 and Phe 134. Numerous van der Waals interactions were also formed by remaining residues (Figure 1(a,b)). Somniferine was found to be another inhibitor with binding energy 9.62 kcal/mol. It forms conventional, carbon and π-donor hydrogen bonding with the residues Leu 141, His 164, Thr 24, Glu 166, Asn 142, Phe 140 and His 163, alkyl and π-alkyl interaction was formed with Cys 145 and His 163. Few van der Waals interactions were formed by remaining residues (Figure 1(c,d)).
Figure 1.
(a) 3 D interaction diagram of Withanoside V with SARS-CoV-2 Mpro, (b) 2 D interaction diagram of Withanoside V with SARS-CoV-2 Mpro, (c) 3 D interaction diagram of Somniferine with SARS-CoV-2 Mpro, (d) 2 D interaction diagram of Somniferine with SARS-CoV-2 Mpro.
Potential inhibitor from T. cordifolia (Giloy) for SARS-CoV-2 Mpro
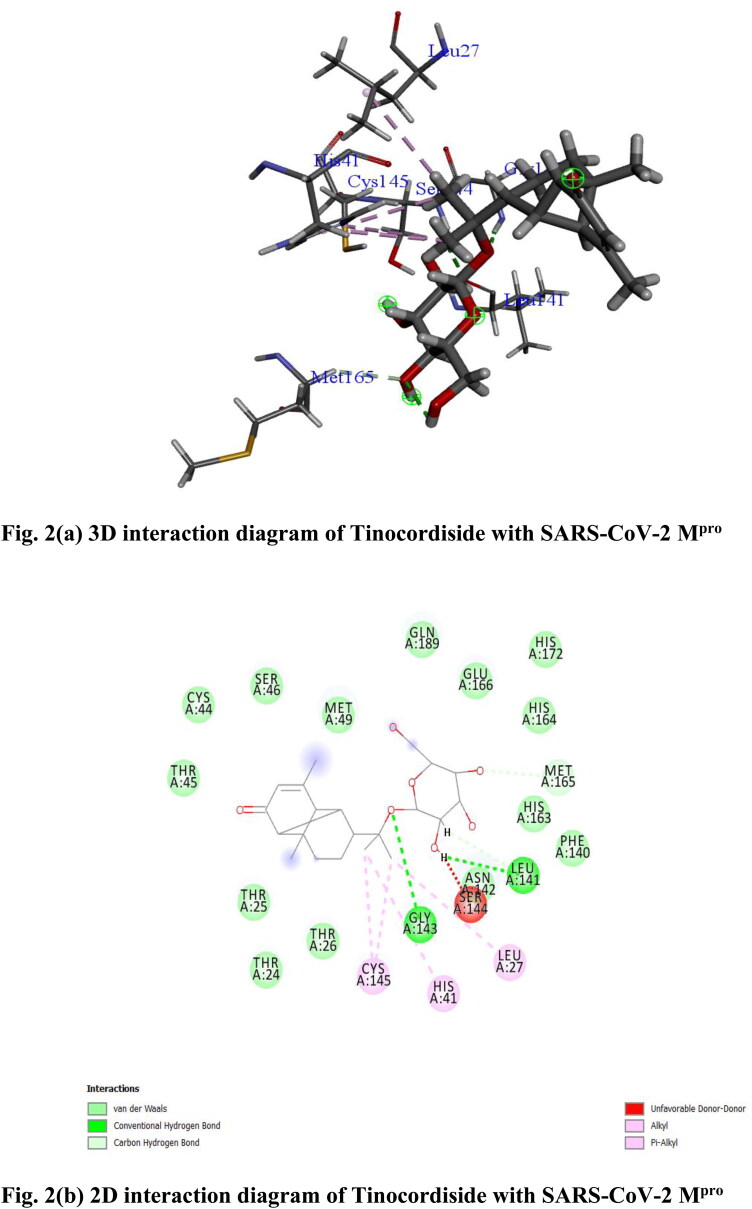
Among 28 active phytochemicals from T. cordifolia (Giloy), only one compound namely Tinocordiside (CID_177384) showed highest binding affinity as compared to built-in ligand N3 for SARS-CoV-2 Mpro as per YASARA scoring. Tinocordiside have binding energy of 8.10 kcal/mol. Tinocordiside is found to be a new reorganized cadinane sesquiterpene glycoside from T. cordifolia (Giloy) (Ghosal & Vishwakarma, 1997). Different 2D–3D interactions formed by Tinocordiside includes conventional and carbon hydrogen bonding with the residues Gly 143, Leu 141 and Met 165, alkyl and π-alkyl interaction were formed with Cys 145, His 41 and Leu 27. Many van der Waals interactions were formed by remaining residues (Figure 2(a,b)).
Figure 2.
(a) 3 D interaction diagram of Tinocordiside with SARS-CoV-2 Mpro, (b) 2 D interaction diagram of Tinocordiside with SARS-CoV-2 Mpro.
Potential inhibitors from O. sanctum (Tulsi) for SARS-CoV-2 Mpro
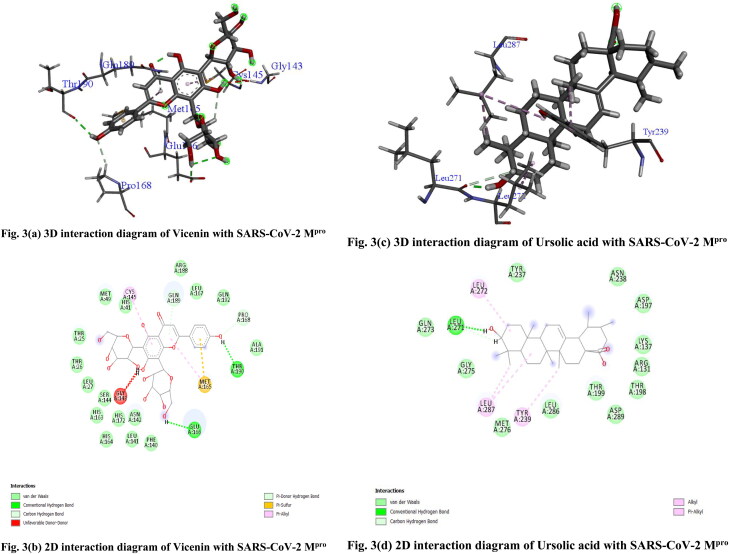
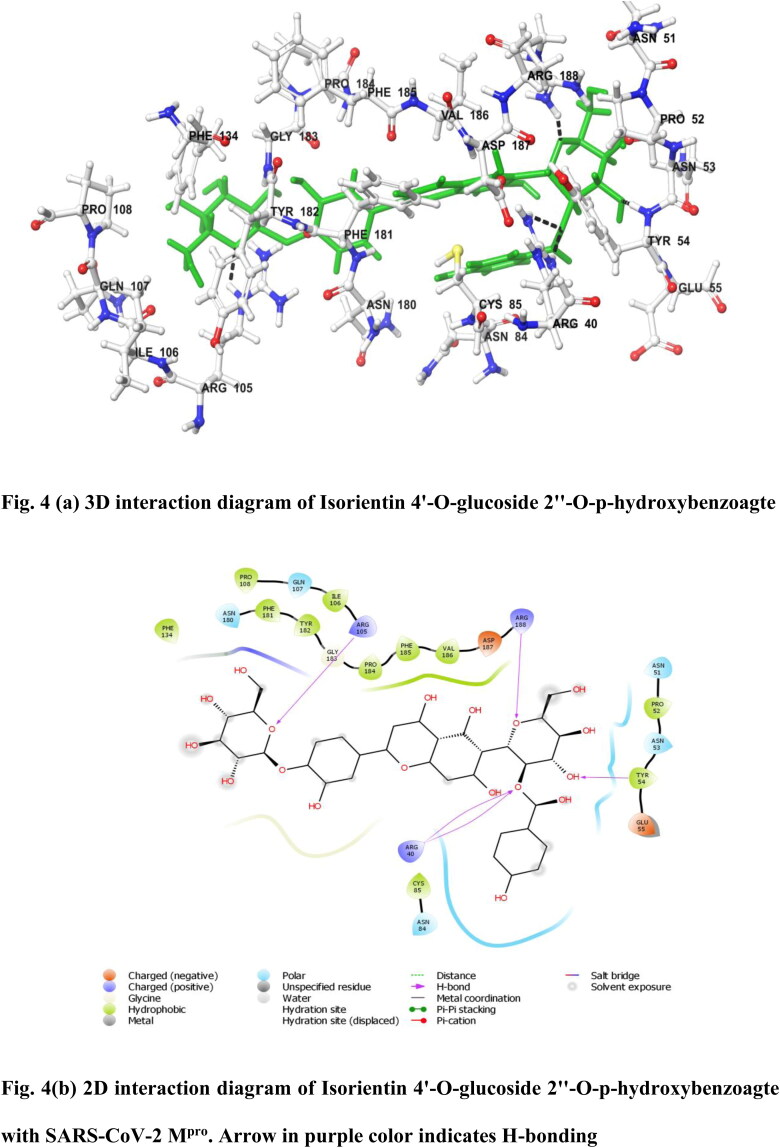
Out of 46 active phytochemicals from O. sanctum (Tulsi), only three compounds namely Vicenin (CID_3084407), Isorientin 4′-O-glucoside 2″-O-p-hydroxybenzoagte (CID_44257986) and Ursolic acid (CID_64945) showed significant binding affinity as compared to built-in ligand N3 for SARS-CoV-2 Mpro. Vicenin was found to have highest binding energy of 8.97 kcal/mol. Vicenin formed many 2D–3D interactions with the receptor protein which includes conventional, carbon and π-donor hydrogen bonding with the residues Glu 166, Thr 190, Gln 189 and Pro 168, a π-sulfur bonding with Met 165, a alkyl interaction was formed with Cys 145 and various van der Waals interactions were found to be formed by remaining residues (Figure 3(a,b)). Second inhibitor was found to be Isorientin 4′-O-glucoside 2″-O-p-hydroxybenzoagte with binding energy 8.55 kcal/mol. It forms different 2D–3D interactions which includes positive charged hydrogen bonding with the residues Arg 40, Arg 105, Arg 188 and hydrophobic interaction with the residue Tyr 54 (Figure 4(a,b)). Another inhibitor was found to be Ursolic acid with binding energy 8.52 kcal/mol. Ursolic acid is a pentacyclic triterpenoid, reported to have anti-viral, anti-inflammatory, antitumor, antimicrobial, anti-hyperlipidemic, anti-ulcer, hepatoprotective, anti-fungal and anti-malarial activities (Prakash & Gupta, 2005; RJPP – Traditional Indian Herbal Plants Tulsi & Its Medicinal Importance & , 2010). It forms conventional and carbon hydrogen bonding with the residue Leu 271, alkyl and π-alkyl interaction with the residues Leu 272, Leu 287 and Tyr 239. It also formed several van der Waals interactions with the remaining residues (Figure 3(c,d)).
Figure 3.
(a) 3 D interaction diagram of Vicenin with SARS-CoV-2 Mpro, (b) 2 D interaction diagram of Vicenin with SARS-CoV-2 Mpro, (c) 3 D interaction diagram of Ursolic acid with SARS-CoV-2 Mpro, (d) 2 D interaction diagram of Ursolic acid with SARS-CoV-2 Mpro.
Figure 4.
(a) 3D interaction diagram of Isorientin 4′-O-glucoside 2″-O-p-hydroxybenzoagte with SARS-CoV-2 Mpro. (b) 2D interaction diagram of Isorientin 4′-O-glucoside 2″-O-p-hydroxybenzoagte with SARS-CoV-2 Mpro. Arrow in purple colour indicates H-bonding.
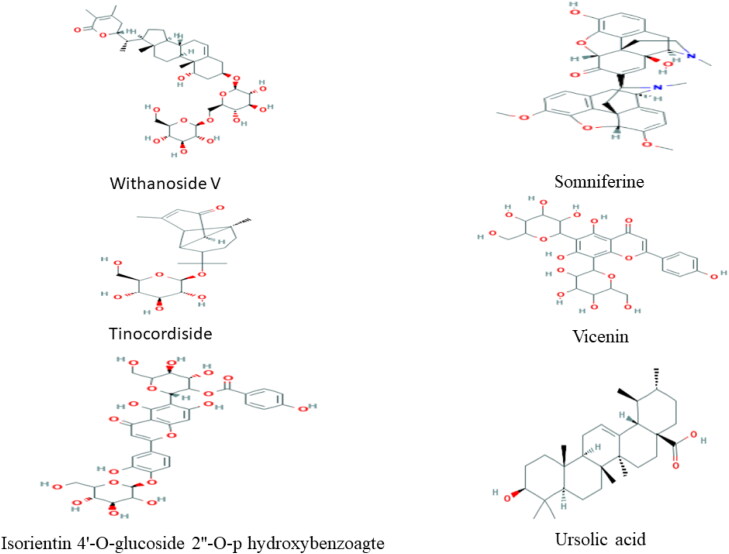
From molecular docking study, as per YASARA scoring, it had been clear that Withanoside V, Somniferine, Tinocordiside, Vicenin, Isorientin 4′-O-glucoside 2″-O-p-hydroxybenzoagte and Ursolic acid from W. somnifera (Ashwagandha), T. cordifolia (Giloy), and O. sanctum (Tulsi) predicted to acts as probable inhibitors of SARS-CoV-2 Mpro. Structures of best docked compounds are shown in Figure 5.
Figure 5.
Structures of best docked compounds Withanoside V, Somniferine, Tinocordiside and Vicenin, Isorientin 4′-O-glucoside 2″-O-p-hydroxybenzoagte and Ursolic acid.
Molecular dynamics simulations
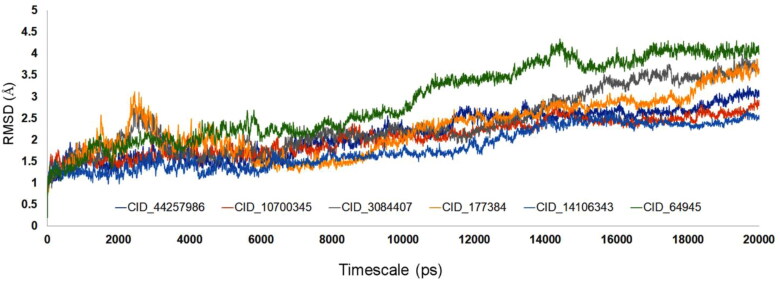
The docked moiety of main protease (Mpro) along with Withanoside V (CID_10700345-b), Somniferine (CID_14106343), Tinocordiside (CID_177384-d), Vicenin (CID_3084407 - c), Isorientin 4′-O-glucoside 2″-O-p-hydroxybenzoagte (CID_44257986-a) and Ursolic acid (CID_64945) was simulated for understanding the structural deviations in the dynamic environment for the timescale of 20 ns. Each complex deviation was recorded for RMSD from its initial position and the values were calculated and plotted simultaneously (Figure 6). While observing the results, it has been found that, RMSD for the compounds shows uniqueness in the dynamic conditions. For the initial 8 ns, except the compound CID_177384 and CID_3084407, all the other compounds showed lower and stable deviations ∼1 to ∼2.5 Å. The compounds CID_177384 and CID_3084407 showed deviations up to ∼3Å, especially from 2 to 4 ns of the MD simulations. After 8 ns, there was visible movement in the upward direction however the stable movement was initiated between ∼ 1.5 and ∼3.5 Å. One of the notable compound, CID_64945 with two hydrogen bond donor and three hydrogen bond acceptor adopts well inside the binding pocket in the docking calculations, and the interactions were stable in the MD simulations. But compound shows much activity with the binding of loop structures in Mpro binding site, and thus the deviations for the CID_64945 was seen from ∼3Å to ∼4Å in between the timescale of 10 to 20 ns of the MD simulations. Another compound CID_177384 (2-(Diethylamino)ethyl tetrahydro-alpha-(1-naphthylmethyl)-2-furanpropionate), holding three oxygen atom along with one nitrogen atom holds the active site binding, but the deviation drift occurs in 18 ns, due to the breaking of hydrogen bonds from the amino acids residues Arg 188 and Gln 189. Interestingly, one of the amino acids residue Glu 166 taking hold at the position of Arg 188 and Gln 189, and this transition makes the sudden drift in the 18–20 ns of the MD simulations. Another compound namely Isorientin 4′-O-glucoside 2″-O-p-hydroxybenzoagte (CID_ 44257986), showed promising stability due to the high allocation of hydrogen bond acceptor atoms, and these atoms are prominent in showing tight binding in the Mpro peptide binding pocket. Even though the compound showed large allocation towards the binding pocket, we did not notice any unbounding in the 20 ns of the MD simulations. The compound Vicenin (CID_3084407) showed similar binding mode of CID_177384, but the drift was seen from 15 to 20 ns, due to strong bond formation with the interactions of residues Glu 166 and Asn 142. The overall RMSD of all the compounds for the timescale of 20 ns is provided in Figure 6.
Figure 6.
RMSD graph of ligand complex with SARS-CoV-2 Mpro for the timescale of 20 ns.
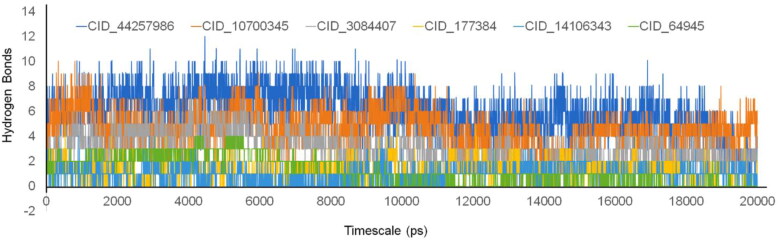
For cognizance the reason of RMSD deviations, we have accounted hydrogen bonds between the protein–ligand complexes (Figure 7). While observing the hydrogen bonds, it has been found that there was a strong correlation between hydrogen bonds and RMSD analysis. It has been noticed that the resemblance of hydrogen bonds getting down after 8 ns, and this had impacted the upwards movement of RMSD from the 9 to 20 ns. In order to understand the contributing amino acids residues, the MD trajectories were carefully analysed. The ligand molecule CID_44257986 showed 8-12 contacts till 10 ns, only after the hydrogen bonds are levelled between 6 and 8 hydrogen bonds. This impact was revealed in the RMSD values, but interestingly one of the amino acid residue Glu 166 was playing vital role in holding the CID_44257986 throughout the MD simulations. The CID_10700345 interaction with Mpro, during the MD simulations, resulted in high residual fluctuations between the regions of 130–190th position. Here also, the amino acid residue Glu 166 was playing vital role in holding the CID_10700345, even though high deviations were seen in residual amino acid positions. The oxygen atom in the ligand CID_10700345 showed 83% of interactions with residue Glu 166 in the MD simulations, which showed significant contribution of Glu 166 in the binding. For the compound CID_3084407, hydrogen bond does not have stability except the Glu 166, but the single amino acid residue pattern holds the CID_3084407 throughout the MD simulations. In this, the other amino acid residue Gln 192, was contributing to hold Gln 166 which form hydrogen bonds with the OH atom of the ligand. The other compound CID_177384 showed tight bound in the binding pocket, but unexpectedly, we did not find a single amino acid residue that holds the ligand throughout 20 ns of the MD simulations. The amino acids residues like Asn 142, Glu 166, Arg 188, Gln 189, Gln 192 were contributing in different intervals for holding the CID_177384 inside the binding pocket. For the compound CID_14106343, the residual movements were not observed high, due to the formation of hydrophobic interactions. Especially the amino acid residues His 41, Met 49 and Met 165 were contributing in hydrophobic interactions with ligand and thus, the ligand is tightly bound inside the binding pocket. For the compound CID_14106343, the key amino acid residue His 41 play role in binding of the ligand. For the compound CID_64945, the amino acid residues provide the ionic bonds, supported by hydrophobic bonds present in the binding pocket. The amino acids residues Asn 119, Gly 143, Asp 187 and Gln 189 were forming the hydrogen bonding interactions. The average hydrogen bonds between the Mpro with all the ligands were provided in Figure S1 (supplementary material), showing the active participation of ligands in tight binding with Mpro.
Figure 7.
Hydrogen bond interactions between the SARS-CoV-2 Mpro and ligand molecules for the timescale of 20 ns.
Drug-likeness and ADMET profile analysis
Drug-likeness test for the best docked compounds was predicted with the help of Lipinski’s rule of five and ADMET molecular property prediction test was performed out by admetSAR server. Lipinski rule of five is a thumb rule of five which aids in differentiating among drug like and non-drug like molecules by obeying its five parameters (Molecular mass, Hydrogen bond donor, Hydrogen bond acceptor, Log P and Molar refractivity); it must obey 2 or more of their parameters. Consequently best docked compounds Withanoside V, Somniferine, Tinocordiside and Vicenin, Isorientin 4′-O-glucoside 2″-O-p-hydroxybenzoagte and Ursolic acid from present study follows more than two parameters of Lipinski rule of five and hence deliberated as drug-like compounds. Additionally, best docked compounds were found to be within the standard scale considering their water solubility (LogS), human intestinal absorption (HIA), Caco-2 permeability and have no carcinogenic effects (Table 2).
4. Discussion
Currently in modern medicine, both individual and combination of anti-malarial, anti-viral and corticosteroid therapy are being used for treating COVID-19. Recently as per WHO Dexamethasone (World Health Organization, 2020) has shown promising results, India based Glen mark has introduced antiviral drug Favipiravir (Glenmark launches Covid-19 drug at Rs 103 per tablet – India news – Hindustan Times, 2020) which has got regulatory approval for Phase III assessment for treating mild to moderate COVID-19 patient. Repurposing of drug for treating COVID-19 is being extensively used for managing the current cases of COVID-19. Natural product with medicinal property can be used, as toxicity profile is less in comparison to synthetic compounds. Consulting Ayurveda for treating COVID-19 provides reliable evidence-based medicinal plants for managing the ailment of respiratory disorders. In silico approach such as molecular docking and molecular dynamic simulation studies provides fundamental starting point in terms of binding energy and stability of ligands with proteins for further research. SARS-CoV-2 Mpro is important in stimulating proteolytic maturation of viral RNA into functional proteins namely RNA polymerase, endoribonuclease and exoribonuclease which also hampers the intrinsic immune system of the host. Therefore, SARS-CoV-2 Mpro can be deliberated as significant target as it helps in translation of polyproteins into individual functional component that subsequently forms 16 NSPs which helps in viral transcription and replication (Jo et al., 2020).
Present work describes the role of active phytoconstituents from Ayurvedic medicinal plants W. somnifera (Ashwagandha), T. cordifolia (Giloy), and O. sanctum (Tulsi) for treating COVID 19 pandemic. As stated earlier in this article, from various studies (Balkrishna et al., 2020; Dharmendra & Deepak, 2020; Varshney et al., 2020) some of the phytoconstituents from these medicinal plants have shown promising effect against COVID-19 targets. Present work reports phytochemicals which are different from previous studies for inhibition of Mpro with significant binding affinity as per YASARA scoring. Six phytochemicals from present study namely Withanoside V, Somniferine, Tinocordiside, Vicenin, Isorientin 4′-O-glucoside 2″-O-p-hydroxybenzoagte and Ursolic acid obtained from medicinal plants, have predicted significant binding energy as compared to the native ligand N3 (Figures 1–4, Table 1). The binding of these identified active phytochemicals with Mpro decreases the process of viral transcription and replication by down turning the cleavage of poly proteins that release NSPs. From present work, it has been suggested that the best docked compounds obtained from Ayurvedic medicinal plants W. somnifera (Ashwagandha), T. cordifolia (Giloy) and O. sanctum (Tulsi) could be predicted to serve as potential inhibitors of SARS-CoV-2 Mpro, with their significant binding affinity, stable MD runs, ADMET prediction and drug-likeness properties. These phytoconstituents not only impede the interaction of viral protein to the host cell to transmit and propagate inside the human body but they are also safe to repurpose against COVID-19 without any toxicity.
Conclusions
COVID-19 is an evolving disease triggered by COVID-19 (SARS-CoV-2) virus and has turned into a global pandemic in the blink of eyes. SARS-CoV-2 Mpro is shown to be highly potent and vital target for the inhibition of COVID-19 contamination. In present study, molecular docking analysis identified six phytochemicals (Withanoside V [10.32 kcal/mol], Somniferine [9.62 kcal/mol], Tinocordiside [8.10 kcal/mol], Vicenin [8.97 kcal/mol], Isorientin 4′-O-glucoside 2″-O-p-hydroxybenzoagte [8.55 kcal/mol] and Ursolic acid [8.52 kcal/mol]) from medicinal plants with potential inhibition and high affinities towards SARS-CoV-2 Mpro, predicted to restrain the action of SARS-CoV-2 Mpro thereby obstructing further translation of viral protein that assists in damaging the vital organs of the host. These phytochemicals might be repurposed against COVID-19. The best docked compounds with drug-like property have harmless ADMET profile which may help in developing optimised potent COVID-19 inhibitors. During MD runs, the trajectories analysis of the studied complexes displayed structural stability. From this study, we may conclude that above stated active phytochemicals, predicted to have the potential to be repurposed as anti-COVID-19 Ayurvedic therapeutics.
Contribution to the field
This contemporary work is executed with the aim of presenting natural phytoconstituents obtained from Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy), Ocimum sanctum (Tulsi) as a remedial option against COVID-19 a global plague.
The existing strategies across the nations are reutilization of medicine for effective treatment and management of COVID-19. Here, natural products play a pivotal role for repurposing against this pandemic as it offers minimal and in some cases no toxicity. As the clock is ticking and earth is under lockdown, in silico studies and virtual analysis may provide evidence based management of COVID-19. It is supported by molecular docking and molecular dynamics simulation analysis of the above mentioned active phytoconstituents against the most utilized macromolecular target of SARS-CoV-2, i.e. main protease (Mpro) which is found to be responsible for growth of virus via proteolytic cleavage, further acting as a transit point for virus entry and replication. Targeting main protease with natural phytoconstituents would serve as checkpoint for viral entry, thereby will resist their further replication and propagation. We hope with this study one could utilize the holistic approach of Ayurvedic medicinal plant to avenge against the grimmest disease of this modern day era.
Supplementary Material
Acknowledgements
BHU administration, Center for Bioinformatics, School of Biotechnology, Department of Biotechnology, ISc, BHU, Varanasi, for the use of YASARA software. We are thankful to Manish Kumar Tripathi, Research associate, Department of Biophysics, AIIMS, New Delhi, for help in image visualization. CS and SKS thankfully acknowledge RUSA-Phase 2.0 Policy (TNmulti-Gen), Dept. of Edn, Govt. of India (Grant No: F.24-51/2014-U).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Anand, K., Ziebuhr, J., Wadhwani, P., Mesters, J. R., & Hilgenfeld, R. (2003). Coronavirus main proteinase (3CLpro) structure: Basis for design of anti-SARS drugs. Science (New York, N.Y.), 300(5626), 1763–1767. 10.1126/science.1085658 [DOI] [PubMed] [Google Scholar]
- Balkrishna, A., Pokhrel, S., Singh, J., & Varshney, A. (2020). Withanone from Withania somnifera may inhibit novel coronavirus (COVID-19) entry by disrupting interactions between viral S-protein receptor binding domain and host ACE2 receptor. Virology Journal. 10.21203/RS.3.RS-17806/V1 [DOI] [Google Scholar]
- Bowers, K. J., Chow, E., Xu, H., Dror, R. O., Eastwood, M. P., Gregersen, B. A., … Shaw, D. E. (2006). Scalable algorithms for molecular dynamics simulations on commodity clusters [Paper presentation]. Proceedings of the 2006 ACM/IEEE Conference on Supercomputing, SC’06, 84. 10.1145/1188455.1188544 [DOI]
- Chan, J. F. W., Li, K. S. M., To, K. K. W., Cheng, V. C. C., Chen, H., & Yuen, K. Y. (2012). Is the discovery of the novel human betacoronavirus 2c EMC/2012 (HCoV-EMC) the beginning of another SARS-like pandemic? The Journal of Infection, 65(6), 477–489. 10.1016/j.jinf.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary, A., Sharma, S., Mittal, A., Gupta, S., & Dua, A. (2020). Phytochemical and antioxidant profiling of Ocimum sanctum. Journal of Food Science and Technology, 1–12. 10.1007/s13197-020-04417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, F., Li, W., Zhou, Y., Shen, J., Wu, Z., Liu, G., Lee, P. W., & Tang, Y. (2012). AdmetSAR: A comprehensive source and free tool for assessment of chemical ADMET properties. Journal of Chemical Information and Modeling, 52(11), 3099–3105. 10.1021/ci300367a [DOI] [PubMed] [Google Scholar]
- Chow, E., Rendleman, C. A., Bowers, K. J., Dror, R. O., Hughes, D. H., Gullingsrud, J., … Shaw, D. E. (2008). Desmond performance on a cluster of multicore processors hardware and operating environment benchmark systems and simulation parameters.
- De Oliveira, O. V., Rocha, G. B., Paluch, A. S., & Costa, L. T. (2020). Repurposing approved drugs as inhibitors of SARS-CoV-2 S-protein from molecular modeling and virtual screening. Journal of Biomolecular Structure & Dynamics, 1–14. 10.1080/07391102.2020.1772885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar, R. S., Verma, V., Suri, K. A., Sangwan, R. S., Satti, N. K., Kumar, A., Tuli, R., & Qazi, G. N. (2006). Phytochemical and genetic analysis in selected chemotypes of Withania somnifera. Phytochemistry, 67(20), 2269–2276. 10.1016/j.phytochem.2006.07.014 [DOI] [PubMed] [Google Scholar]
- Dharmendra, M., & Deepak, S. (2020). Evaluation of traditional ayurvedic preparation for prevention and management of the novel coronavirus (SARS-CoV-2) using molecular docking approach. ChemRxiv, 10.26434/chemrxiv.12110214.v1. [DOI] [Google Scholar]
- Elmezayen, A. D., Al-Obaidi, A., Şahin, A. T., & Yelekçi, K. (2020). Drug repurposing for coronavirus (COVID-19): in silico screening of known drugs against coronavirus 3CL hydrolase and protease enzymes. Journal of Biomolecular Structure and Dynamics, 1–13. 10.1080/07391102.2020.1758791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly, B., Kumar, N., Ahmad, A. H., & Rastogi, S. K. (2018). Influence of phytochemical composition on in vitro antioxidant and reducing activities of Indian ginseng [Withania somnifera (L.) Dunal] root extracts. Journal of Ginseng Research, 42(4), 463–469. 10.1016/j.jgr.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal, S., & Vishwakarma, R. A. (1997). Tinocordiside, a new rearranged cadinane sesquiterpene glycoside from Tinospora cordifolia. Journal of Natural Products, 60(8), 839–841. 10.1021/np970169z [DOI] [Google Scholar]
- Glenmark launches Covid-19 drug at Rs 103 per tablet – India news – Hindustan Times (2020). Retrieved June 24, 2020, from https://www.hindustantimes.com/india-news/glenmark-launches-covid-19-drug-after-dcgi-nod/story-kwOjjcAbv7zQ1Sksw8qXbP.html
- Jamshidi, N., & Cohen, M. M. (2017). The clinical efficacy and safety of tulsi in humans: A systematic review of the literature. Evidence-Based Complementary and Alternative Medicine, 2017, 9217567. 10.1155/2017/9217567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram, B., Singh, T., Mukherjee, G., Mathur, A., Shekhar, S., & Shekhar, V. (2012). Sanjeevini: A freely accessible web-server for target directed lead molecule discovery. BMC Bioinformatics, 13 (Suppl 17), S7. 10.1186/1471-2105-13-S17-S7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo, S., Kim, S., Shin, D. H., & Kim, M. S. (2020). Inhibition of SARS-CoV 3CL protease by flavonoids. Journal of Enzyme Inhibition and Medicinal Chemistry, 35(1), 145–151. 10.1080/14756366.2019.1690480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns Hopkins CSSE (2020). Coronavirus COVID-19 (2019-nCoV). Retrieved July 18, 2020, from Coronavirus COVID-19 Global Cases by Johns Hopkins CSSE website: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6
- Kapoor, L. (1990). CRC handbook of ayurvedic medicinal plants/author, L.D. Kapoor. – Version details – Trove. Boca Raton, FL: CRC Press. Retrieved June 24, 2020, from https://trove.nla.gov.au/work/16589020?q&versionId=19467951
- Khan, R. J., Jha, R. K., Amera, G. M., Jain, M., Singh, E., Pathak, A., … Singh, A. K. (2020). Targeting SARS-CoV-2: A systematic drug repurposing approach to identify promising inhibitors against 3C-like proteinase and 2′-O-ribose methyltransferase. Journal of Biomolecular Structure and Dynamics, 1–14. 10.1080/07391102.2020.1753577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, S. A., Zia, K., Ashraf, S., Uddin, R., & Ul-Haq, Z. (2020). Identification of chymotrypsin-like protease inhibitors of SARS-CoV-2 via integrated computational approach. Journal of Biomolecular Structure and Dynamics, 1–10. 10.1080/07391102.2020.1751298. [DOI] [PubMed] [Google Scholar]
- Krieger, E., Vriend, G., & Kelso, J. (2014). YASARA view – Molecular graphics for all devices – From smartphones to workstations. Bioinformatics (Oxford, England), 30(20), 2981–2982. 10.1093/bioinformatics/btu426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski, C. A. (2004). Lead- and drug-like compounds: The rule-of-five revolution. Drug Discovery Today. Technologies, 1(4), 337–341. 10.1016/j.ddtec.2004.11.007 [DOI] [PubMed] [Google Scholar]
- Matsuda, H., Murakami, T., Kishi, A., & Yoshikawa, M. (2001). Structures of withanosides I, II, III, IV, V, VI, and VII, new withanolide glycosides, from the roots of Indian Withania somnifera DUNAL. and inhibitory activity for tachyphylaxis to clonidine in isolated guinea-pig ileum. Bioorganic & Medicinal Chemistry, 9(6), 1499–1507. 10.1016/S0968-0896(01)00024-4 [DOI] [PubMed] [Google Scholar]
- Mengist, H. M., Fan, X., & Jin, T. (2020). Designing of improved drugs for COVID-19: Crystal structure of SARS-CoV-2 main protease Mpro. Signal Transduction and Targeted Therapy, 5(1), 1–2. 10.1038/s41392-020-0178-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal, L., Kumari, A., Srivastava, M., Singh, M., & Asthana, S. (2020). Identification of potential molecules against COVID-19 main protease through structure-guided virtual screening approach. Journal of Biomolecular Structure and Dynamics, 1–26. 10.1080/07391102.2020.1768151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothay, D., & Ramesh, K. V. (2020). Binding site analysis of potential protease inhibitors of COVID-19 using AutoDock. VirusDisease, 31(2), 194–196. 10.1007/s13337-020-00585-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee, R., Dash, P. K., & Ram, G. C. (2005). Immunotherapeutic potential of Ocimum sanctum (L) in bovine subclinical mastitis. Research in Veterinary Science, 79(1), 37–43. 10.1016/j.rvsc.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Muralidharan, N., Sakthivel, R., Velmurugan, D., & Gromiha, M. M. (2020). Computational studies of drug repurposing and synergism of lopinavir, oseltamivir and ritonavir binding with SARS-CoV-2 protease against COVID-19. Journal of Biomolecular Structure and Dynamics, 1–6. 10.1080/07391102.2020.1752802 [DOI] [PubMed] [Google Scholar]
- Nadkarni, K. M., & Krishnarao, M. (1954). Dr. K. M. Nadkarni’s Indian materia medica, with Ayurvedic, Unani-Tibbi, Siddha, Allopathic, Homeopathic, Naturopathic & home remedies, appendices & indexes/originally. Retrieved June 24, 2020, from https://trove.nla.gov.au/work/18643838?q&versionId=21887031
- Panchabhai, T. S., Kulkarni, U. P., & Rege, N. N. (2008). Validation of therapeutic claims of Tinospora cordifolia: A review. Phytotherapy Research: PTR, 22(4), 425–441. 10.1002/ptr.2347 [DOI] [PubMed] [Google Scholar]
- Policy | Ministry of Health and Family Welfare | GOI. (2020). Retrieved February 8, 2020, from https://mohfw.gov.in/documents/policy
- Prakash, P., Gupta, N. (2005). Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: A short review. Retrieved June 24, 2020, from Indian Journal of Physiology and Pharmacology website https://pubmed.ncbi.nlm.nih.gov/16170979/ [PubMed]
- RJPP – Traditional Indian Herbal Plants Tulsi and Its Medicinal Importance (2010). Retrieved June 24, 2020, from http://rjpponline.org/AbstractView.aspx?PID=2010-2-2-1
- Selvaraj, C., Sakkiah, S., Tong, W., & Hong, H. (2018). Molecular dynamics simulations and applications in computational toxicology and nanotoxicology. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 112, 495–506. 10.1016/j.fct.2017.08.028 [DOI] [PubMed] [Google Scholar]
- Sharma, P., Dwivedee, B. P., Bisht, D., Dash, A. K., & Kumar, D. (2019). The chemical constituents and diverse pharmacological importance of Tinospora cordifolia. Heliyon, 5(9), e02437. 10.1016/j.heliyon.2019.e02437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, P., Parmar, J., Sharma, P., Verma, P., & Goyal, P. K. (2011). Radiation-induced testicular injury and its amelioration by tinospora cordifolia (an Indian medicinal plant) extract. Evidence-Based Complementary and Alternative Medicine: ECAM, 2011, 643847. 10.1155/2011/643847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, S. (1999). Bioactive botanicals from Basil (Ocimum sp.). Journal of Scientific & Industrial Research, 58, 332.–. [Google Scholar]
- Sharma, U., Bala, M., Kumar, N., Singh, B., Munshi, R. K., & Bhalerao, S. (2012). Immunomodulatory active compounds from Tinospora cordifolia. Journal of Ethnopharmacology, 141(3), 918–926. 10.1016/j.jep.2012.03.027 [DOI] [PubMed] [Google Scholar]
- Singh, R. H., Narsimhamurthy, K., & Singh, G. (2008). Neuronutrient impact of Ayurvedic Rasayana therapy in brain aging. Biogerontology, 9(6), 369–374. 10.1007/s10522-008-9185-z [DOI] [PubMed] [Google Scholar]
- Thatte, U. M., Kulkarni, M. R., & Dahanukar, S. A. (1992). Immunotherapeutic modification of Escherichia coli peritonitis and bacteremia by Tinospora cordifolia. Journal of Postgraduate Medicine, 38(1), 13–15. [PubMed] [Google Scholar]
- Trott, O., & Olson, A. J. (2009). AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry, 31(2), NA. 10.1002/jcc.21334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney, K. K., Varshney, M., Nath, B. (2020). Molecular Modeling of Isolated Phytochemicals from Ocimum sanctum Towards Exploring Potential Inhibitors of SARS Coronavirus Main Protease and Papain-Like Protease to Treat COVID-19. Retrieved from https://papers.ssrn.com/abstract=3554371
- Ven Murthy, M. R., Ranjekar, P. K., Ramassamy, C., & Deshpande, M. (2010). Scientific basis for the use of Indian ayurvedic medicinal plants in the treatment of neurodegenerative disorders: Ashwagandha. Central Nervous System Agents in Medicinal Chemistry, 10(3), 238–246. 10.2174/1871524911006030238 [DOI] [PubMed] [Google Scholar]
- Vyas, V. K., Bhandari, P., & Patidar, R. (2011). A comprehensive review on Withania somnifera dunal. Journal of Natural Remedies, 11(1), 1–13. 10.18311/JNR/2011/43 [DOI] [Google Scholar]
- Walls, A. C., Park, Y. J., Tortorici, M. A., Wall, A., McGuire, A. T., & Veesler, D. (2020). Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell, 181(2), 281–292.e6. 10.1016/j.cell.2020.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L., Wang, Y., Ye, D., & Liu, Q. (2020). Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. International Journal of Antimicrobial Agents, 55(6), 105948–105948. 10.1016/j.ijantimicag.2020.105948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson, E. M. (2002). Major herbs of ayurveda. Retrieved from https://agris.fao.org/agris-search/search.do?recordID=US201300080597
- World Health Organization (2020). WHO welcomes preliminary results about dexamethasone use in treating critically ill COVID-19 patients. Retrieved June 24, 2020, from World Health Organization website: https://www.who.int/news-room/detail/16-06-2020-who-welcomes-preliminary-results-about-dexamethasone-use-in-treating-critically-ill-covid-19-patients
- World Health Organization (WHO) (2020). Coronavirus disease (COVID-19) situation report-141 situation in numbers (by WHO Region). In Data as received by WHO from national authorities by 10:00 CEST, 13 May 2020. Retrieved from https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200513-covid-19-sitrep-114.pdf?sfvrsn=17ebbbe_4
- Wrapp, D., Wang, N., Corbett, K. S., Goldsmith, J. A., Hsieh, C.-L., Abiona, O., … McLellan, J. S. (2020). Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. BioRxiv : The Preprint Server for Biology, 10.1101/2020.02.11.944462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav, R., Selvaraj, C., Aarthy, M., Kumar, P., Kumar, A., Singh, S. K., & Giri, R. (2020). Investigating into the molecular interactions of flavonoids targeting NS2B-NS3 protease from ZIKA virus through in-silico approaches. Journal of Biomolecular Structure and Dynamics, 10.1080/07391102.2019.1709546 [DOI] [PubMed] [Google Scholar]
- Ye, Z. W., Yuan, S., Yuen, K. S., Fung, S. Y., Chan, C. P., & Jin, D. Y. (2020). Zoonotic origins of human coronaviruses. International Journal of Biological Sciences, 16(10), 1686–1697. 10.7150/ijbs.45472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, S., Chan, H. C. S., & Hu, Z. (2017). Using PyMOL as a platform for computational drug design. WIREs Computational Molecular Science, 7(2), e1298. 10.1002/wcms.1298 [DOI] [Google Scholar]
- Zhang, L., Lin, D., Sun, X., Curth, U., Drosten, C., Sauerhering, L., Becker, S., Rox, K., & Hilgenfeld, R. (2020). Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science (New York, N.Y.), 368(6489), 409–412. 10.1126/science.abb3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, N., Zhang, D., Wang, W., Li, X., Yang, B., Song, J., Zhao, X., Huang, B., Shi, W., Lu, R., Niu, P., Zhan, F., Ma, X., Wang, D., Xu, W., Wu, G., Gao, G. F., & Tan, W., China Novel Coronavirus Investigating and Research Team (2020). A novel coronavirus from patients with pneumonia in China, 2019. The New England Journal of Medicine, 382(8), 727–733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla, A., Chan, J. F. W., Azhar, E. I., Hui, D. S. C., & Yuen, K. Y. (2016). Coronaviruses – Drug discovery and therapeutic options. Nature Reviews. Drug Discovery, 15(5), 327–347. 10.1038/nrd.2015.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.