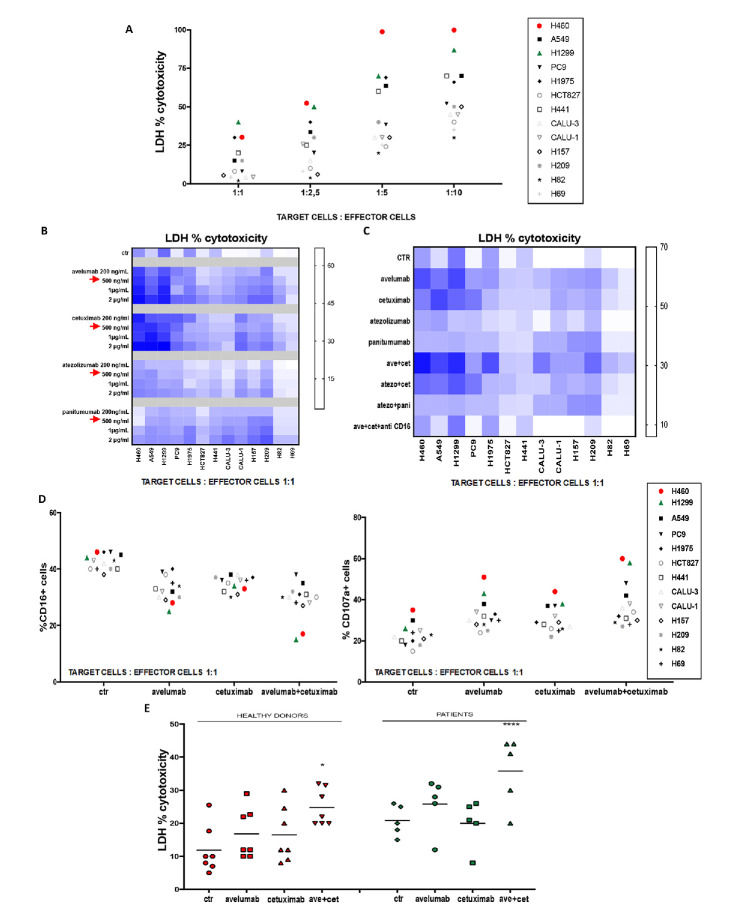
Figure 1.
In vitro evidence of natural killers (NK) activation and cytotoxic lytic activity following cetuximab and/or avelumab treatment. (A) Lactate dehydrogenase (LDH) cytotoxicity assay was performed on 13 lung cell lines, which were used as target cells, and were cocultured with NK cells from healthy donors (HD), which were used as effector cells. Different target: effector cell ratios were used to test cancer cell cytotoxicity. (B) Heat map graph representation of LDH cytotoxicity assay, which was performed on 13 lung cancer cell lines. Cancer cell lines were treated with different doses of avelumab, cetuximab, atezolizumab or panitumumab, as indicated. Intensity of the blue colour indicates the degree of LDH release, as reported on the quantitative scale on the right. (C) Heat map graph representation of LDH cytotoxicity assay, which was performed on 13 lung cell lines that were cocultured with NK cells isolated from HD in a 1:1 ratio and with: avelumab, cetuximab, atezolizumab or panitumumab alone or in combination. Treatments with avelumab and/or cetuximab were also performed in the presence of an anti-CD16 blocking antibody. Intensity of the blue colour indicates the degree of LDH release, as reported on the quantitative scale on the right. (D) Flow cytometric analysis of CD16 (left panel) and CD107A (right panel) expression on NK cells from HD (effector cells), which were cocultured with different human cancer cell lines (target cells), in a 1:1 ratio. (E) LDH cytotoxicity assay was performed on supernatants obtained from cocultures of the H1299 lung cancer cell line (as target cells) and of NK cells which were obtained from seven HD or from five NSCLC patients (as effector cells), in a 1:1 ratio. H1299 cells were treated for 24 hours with avelumab, cetuximab or the combination of both drugs. After 24 hours, NK cells were added and cocultured for 4 hours. *p≤0.05; **p≤0.01; ***p≤0.001; ****p≤0.0001 by one-way ANalysis Of VAriance (ANOVA) test.