Fig. 2.
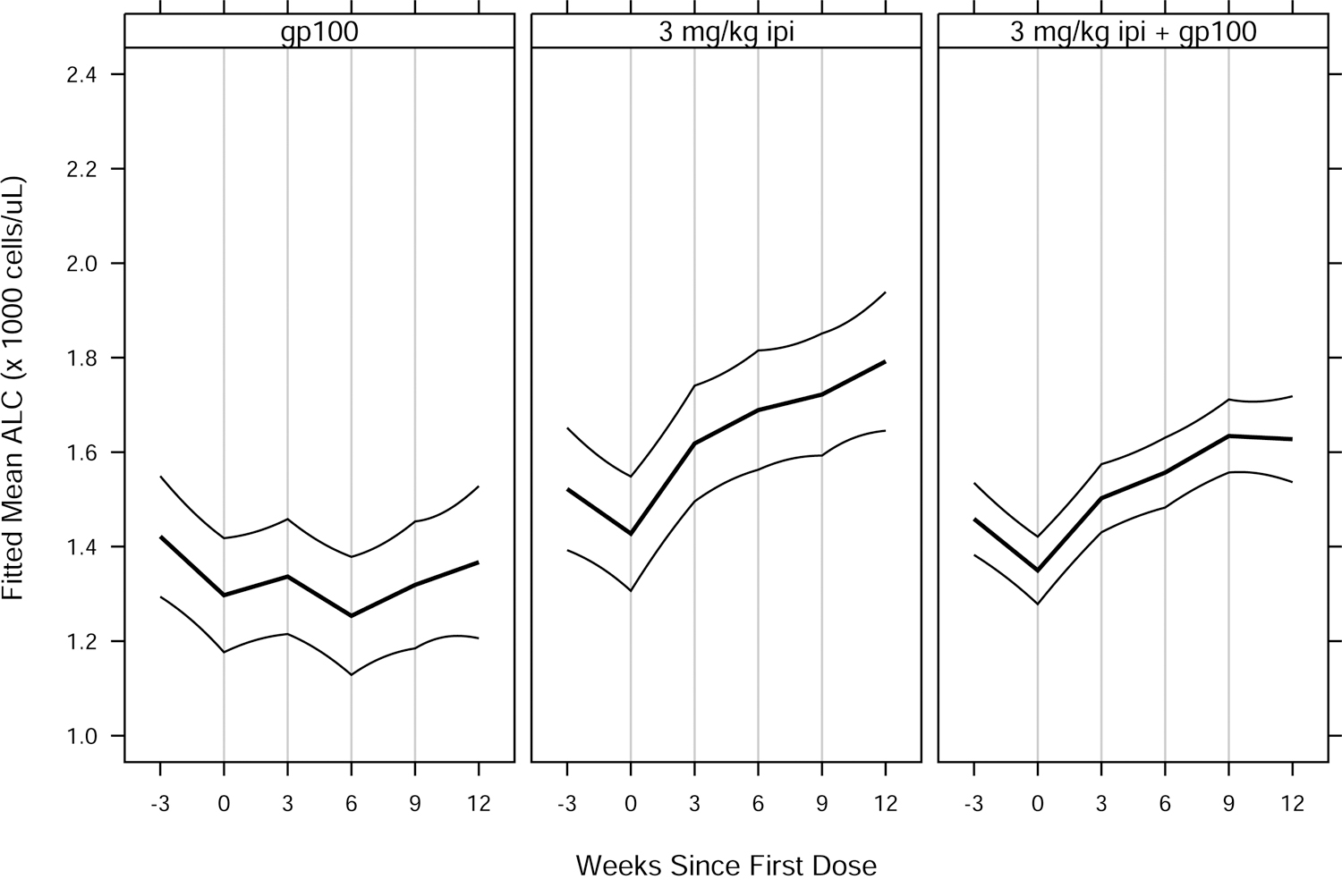
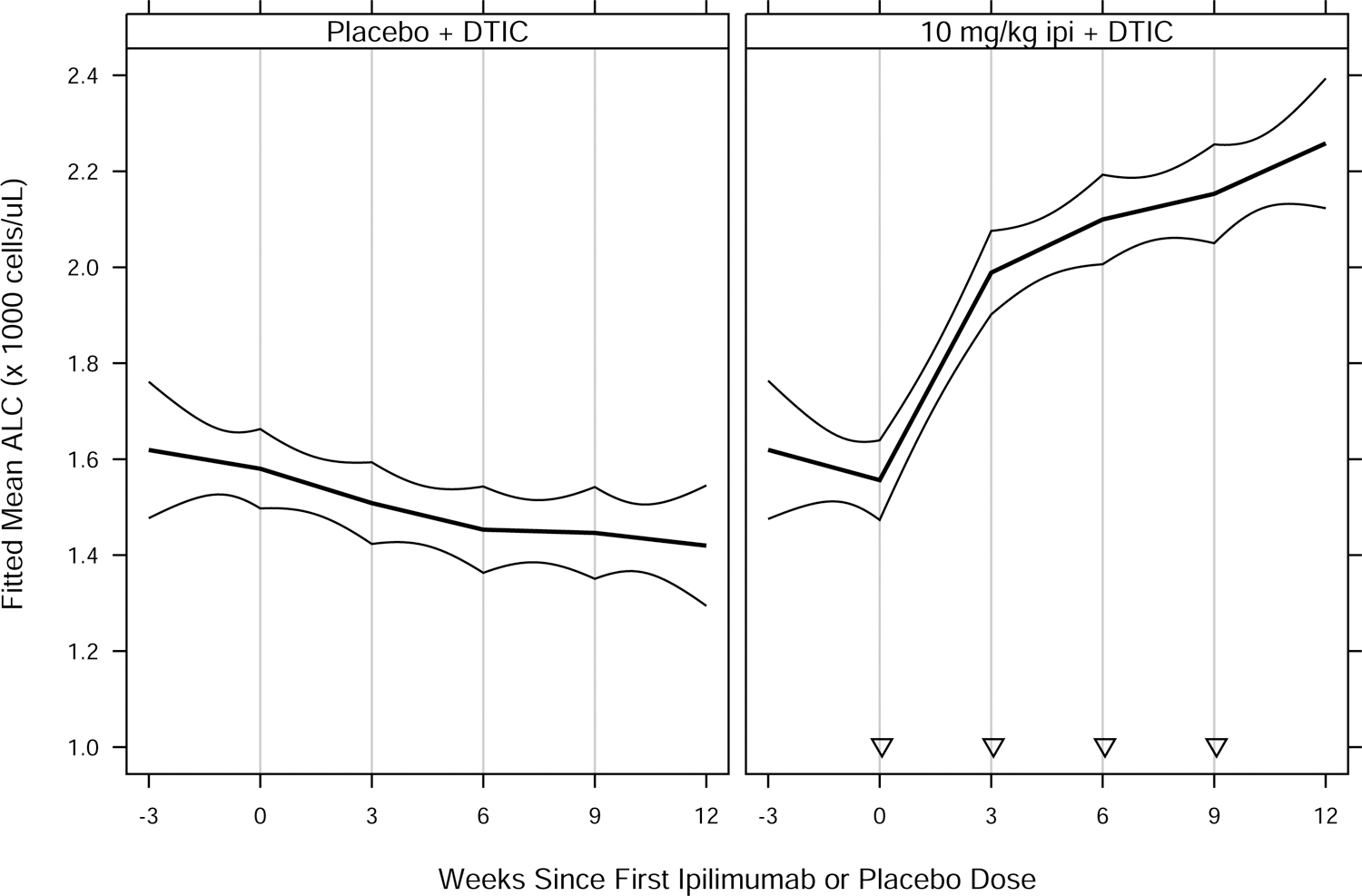
Fitted mean ALC vs. weeks since first ipilimumab or placebo dose, by treatment group for (A) MDX010–20; and (B) CA184–024. Thick curves show fitted means and thin curves show pointwise two-sided 95% confidence intervals for the means. Vertical lines show nominal dosing days. Inverted triangles in (B) indicate ipilimumab doses, in contrast to placebo doses. Patients included in the analyses — 639 of 643 (MDX010–20) and 497 of 498 (CA184–024) — had ≥ 1 ALC assessment in the period from 28 days prior to a confirmed date of treatment initiation to 28 days after dose 4 of study drug. For each study, estimated means and confidence intervals are from an extended linear model, fit by restricted maximum likelihood. The model had fixed effects of treatment group and days since first dose (DSFD). The effect of DSFD was modeled by linear regression splines with, for each patient, a knot at the actual day of each dose, and interaction between treatment group and the splines. Within-patient correlations were modeled by a spatial exponential structure with Euclidean distance, and within-patient variances were inversely proportional to the number of ALC measurements on a given day. DTIC = dacarbazine; gp100 = glycoprotein 100 peptide vaccine; ipi = ipilimumab.
