| Alendronate (Fosamax) |
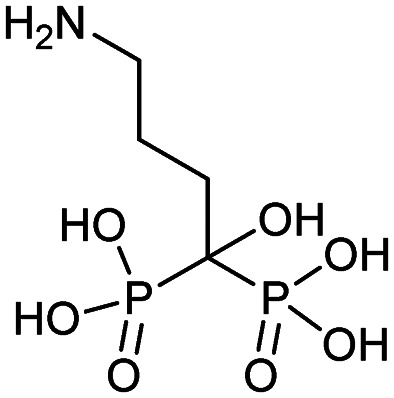
|
11/24/1999 (Merck Research Laboratories) |
Osteogenesis imperfecta in pediatric patients (age >4) |
| Bone manifestations of Gaucher disease |
| Paget's disease |
| Glucocorticoid-induced osteoporosis |
| Osteoporosis |
| Risedronate (Actonel) |
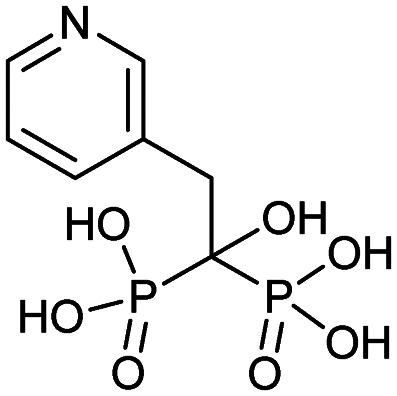
|
3/27/1998 (Procter and Gamble Pharmaceuticals) |
Osteogenesis imperfecta |
| Ibandronate (Boniva) |
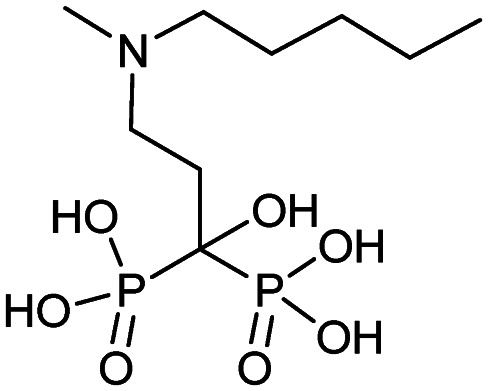
|
5/16/2003 (Hoffmann-La Roche Inc.) |
Osteoporosis in post-menopausal women |
| Pamidronate (Aredia) |
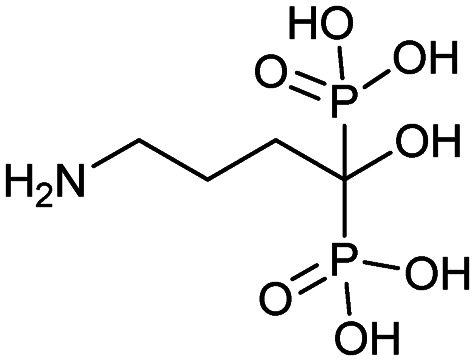
|
9/22/1998 (Novartis) |
Osteoporosis |
| Hypercalcemia |
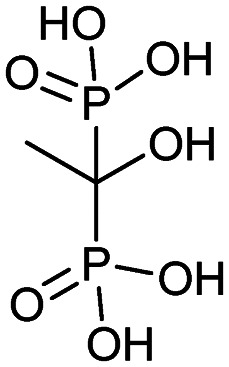
| Etidronate (Didronel) |
|
4/20/1987 (MGI Pharma Inc.) |
Osteoporosis |
| Paget's disease |
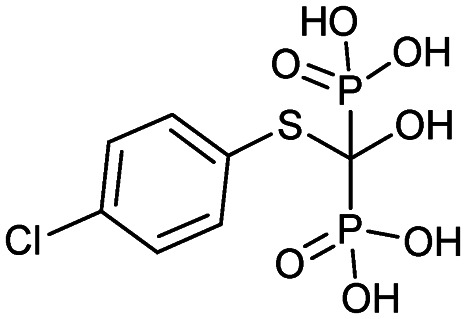
| Tiludronate (Skelid) |
|
3/7/1997 (Sanofi Aventis US) |
Paget's disease |
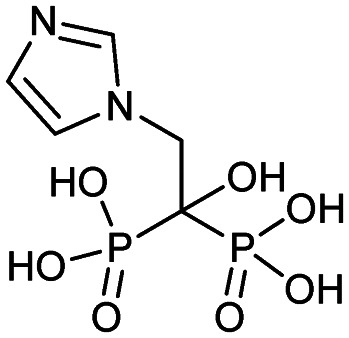
| Zoledronate (Zometa, Reclast) |
|
2/22/2002 (Novartis) |
Tumor-induced hypercalcemia |
| Hypercalcemia of malignancy |







