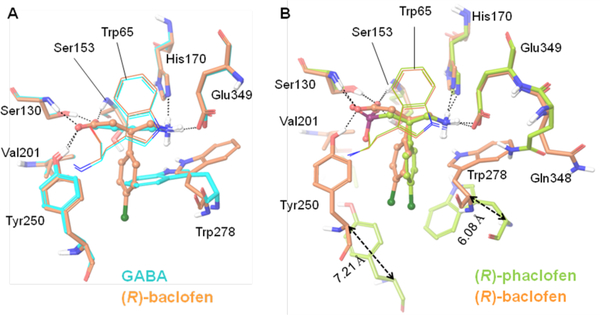
Figure 8.
Binding mode of ligands as shown in X-ray crystal structures.29 (A) Depiction of the differences in binding site residues of GABAB bound to the agonists, GABA (cyan) and (R)-baclofen (orange). The LB2 residue Trp278 is highly flexible, and undergoes rotameric transition to accommodate the bulky substituent p-chlorophenyl of (R)-baclofen. Other residues are conserved. (B) Depiction of the differences in binding site residues of the GABAB bound to the agonist (R)-baclofen (orange) and the antagonist (S)-phaclofen (green). As shown, the LB2 lobe of GBR1b undergoes major changes to govern the closed state of GABAB. The LB2 residues undergo major perturbation, especially Val201, Tyr250, and Trp278, which line the binding site during agonist binding (activation of the GABAB receptor). On the contrary, most of the LB1 residues, except for Gln348, are conserved in the resting (inactive) and active states of the GABAB receptor. Gln348 undergoes rotameric transition to allow a high degree of freedom to Trp278. The LB2 domain of the GBR1b subunit undergoes a major shift during the activation process. For example, the backbone Cαs of Tyr250 and Trp278 move 7.21 and 6.08 Å, respectively, between active and resting (inactive) states. These images were generated using the Schrödinger suite.