Fig. 2. SDF1 activates integrin αvβ3 on the cell surface by binding to site 2 in a CXCR4-independent manner.
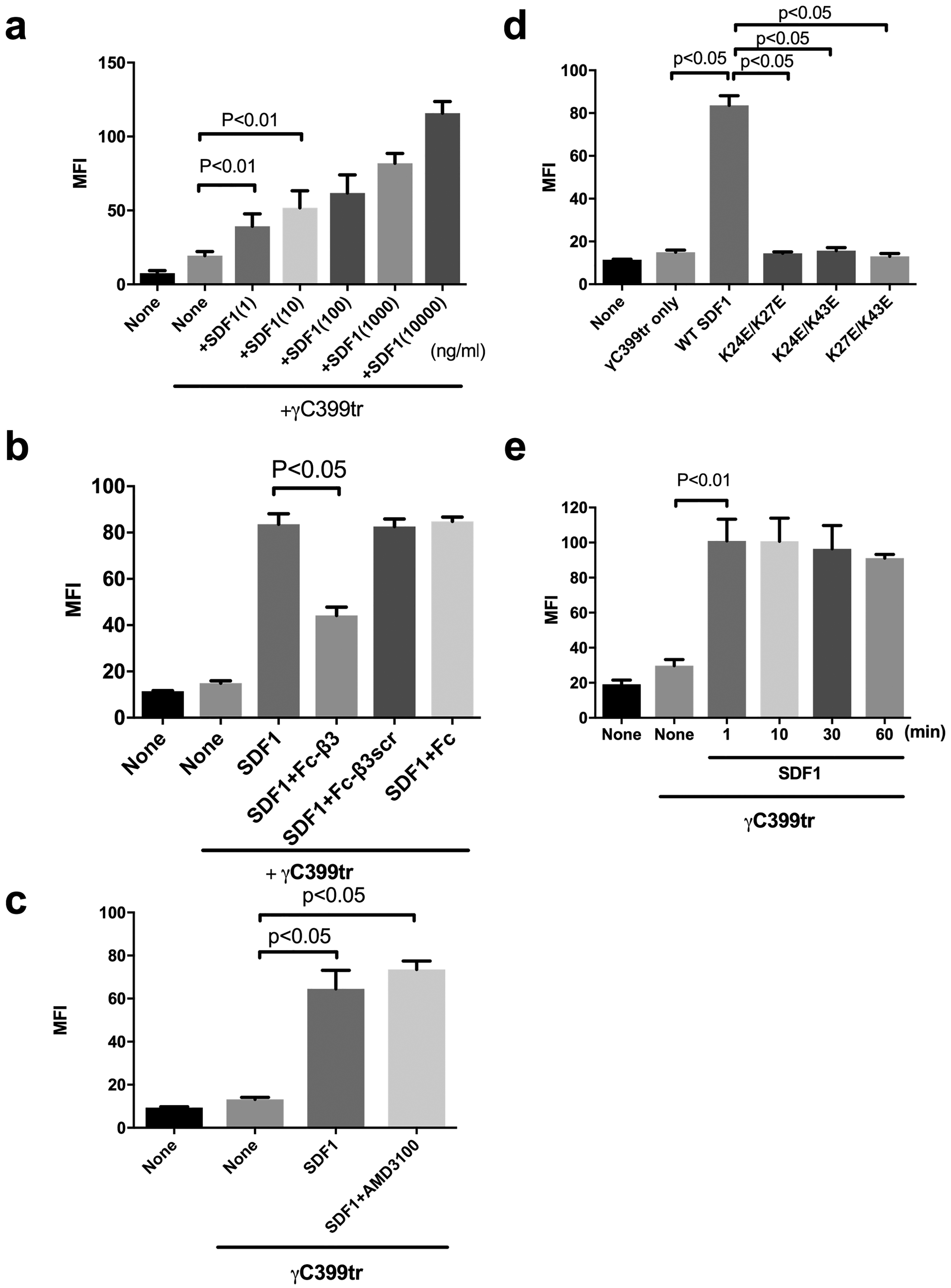
The binding of FITC-labeled γC399tr to the cell-surface αvβ3 on β3-CHO cells in the presence of SDF and Fc-β3 peptide was measured using flow cytometry as described in the methods section. MFI= median fluorescent intensity. Data are shown as means +/− SEM of triplicate experiments. (a) SDF1 activates integrin αvβ3 on β3-CHO cells in a dose-dependent manner (n=4). (b) Fc-β3 peptide, not Fc-β3scr or Fc, suppresses SDF1-mediated activation of integrin αvβ3. (c) AMD3100, a CXCR4 inhibitor, does not affect SDF1-mediated αvβ3 activation in an allosteric manner. (d) SDF1 mutations in the predicted site 2-binding interface of SDF1 are defective in αvβ3 activation. (e) SDF1 fully activates cell-surface αvβ3 in 1 min and the activation lasts for 1 h.
