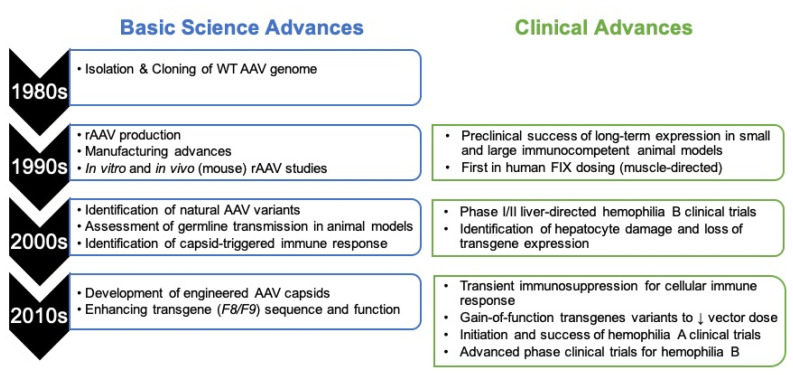
Figure 1.
Timeline of evolution of rAAV from basic science advances to clinical gene therapy.
A significant body of basic science research allowed for translation from wildtype AAV to the recombinant AAV (rAAV) vectors used today. These included improvements in vector engineering and manufacturing, transgene optimization, and elucidating the immune response to rAAV in humans (as no preclinical model predicted this result). Further, laborious efforts in preclinical toxicity evaluation in the early 2000’s allowed clinical trials to advance rapidly in the 2000–2010s without requiring reassessment of these pharmacology/toxicology studies. Clinically, these data have allowed moving from the first-in-human trials in skeletal muscle to phase III trials for both hemophilia A and B with licensure expected in the near future.