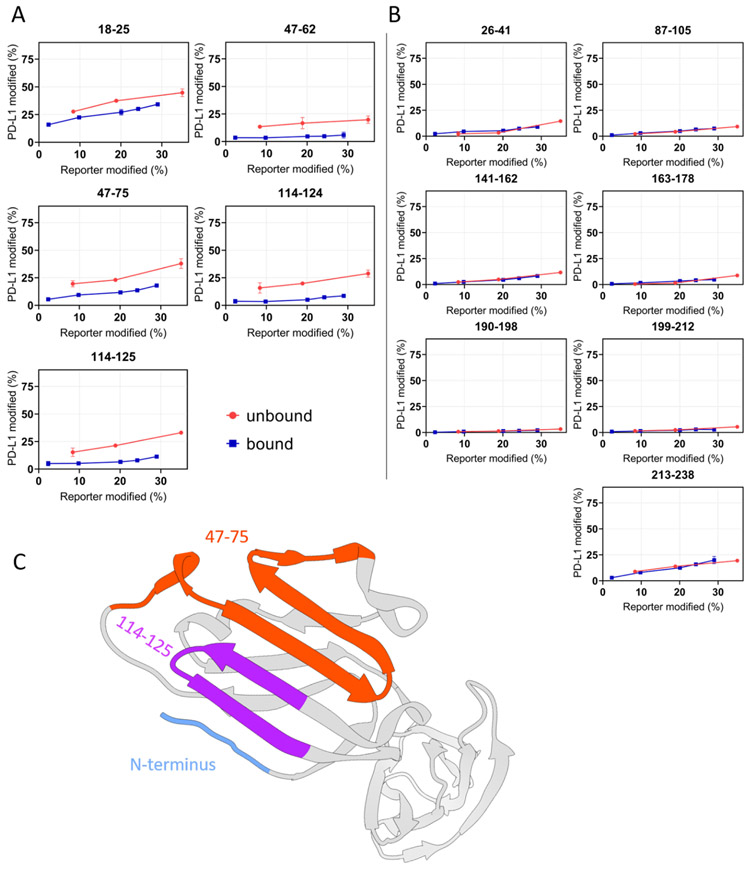
Figure 3. “Time-dependent” FPOP response curves of each peptide of PD-L1.
(A) Differential yields of FPOP observed between macrocycle-bound and unbound PD-L1 (represented by 5 tryptic peptides) suggest three discontinuous binding interfaces on PD-L1, including N-terminus, 47-75, and 114-125. (B) The overlapping of FPOP response curves indicate comparable surface solvent accessibility of these regions between bound and unbound states. These regions are not involved in the macrocycle binding. (C) FPOP results mapped onto the PD-L1 structure (PDB 4Z18) shows comparable outcome as that of HDX. The error bars correspond to ± SD from triplicate measurements and show that for binding sites, the differences between the accumulated differences for three points representing bound and unbound are at least 3 times the propagated errors (sq root of the sum of the squares of the SDs). This corresponds to 99.7% confidence in the assignment.