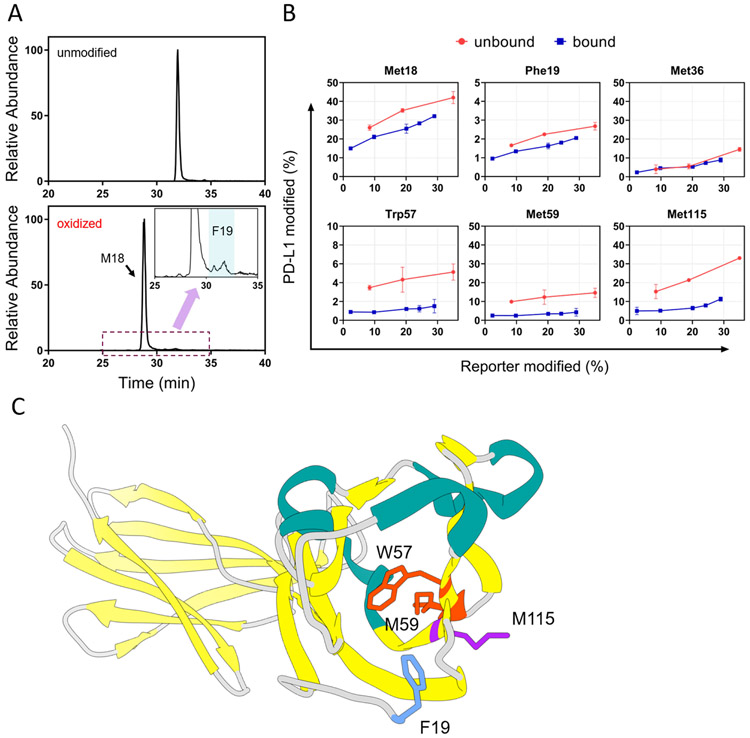
Figure 4.
(A) XICs corresponding to modified and unmodified peptide 18-25 (amino acid sequence MFTVTVPK) showed Met18 is the major modification site, whereas modification on Phe19 is less abundant. The insert represents a zoomed-in region where peptides with Phe19 oxidation elutes. (B) Residue-level time-dependent curves of macrocycle-bound and unbound PD-L1. Residues identified that show significant differential FPOP yields between bound and unbound states of PD-L1 include M18, F19, W57, M59, and M115. Residues were identified by manual interpretation of the product-ion (MS/MS) spectra. Error bars correspond to ± SD from triplicate measurements. The error bars correspond to ± SD from triplicate measurements and show that for binding sites, the differences between the accumulated differences for three points representing bound and unbound are at least 3 times the propagated errors (sq root of the sum of the squares of the SDs). This corresponds to 99.7% confidence in the assignment. (C) Close-up view of these residues show they are spatially localized on the N-lobe of PD-L1.