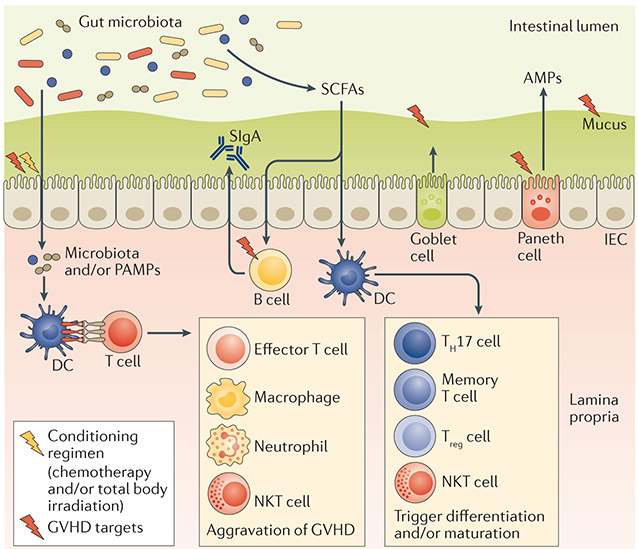
Figure 4 ∣. The interplay between gut microbiota and host physiology and immunity.
The host immune system maintains gut microbiota diversity and prevents outgrowth of pathobionts. Under steady-state conditions, the gut epithelial surface maintains an intact barrier that prevents bacterial invasion into the host tissue; this is accomplished by antimicrobial peptides (AMPs) produced by Paneth cells that create a sterility gradient, mucus produced by goblet cells that separates the microbiota from host epithelial tissue and secretory immunoglobulin A (SIgA) that neutralizes biologically active microbial antigens. Short-chain fatty acids (SCFAs) are bacterial fermentation products. They can be used as an energy source and also regulate the differentiation, recruitment and activation of immune cells. In the setting of allogeneic haematopoietic stem cell transplantation (allo-HSCT), total body irradiation and chemotherapy reduce the integrity of the intestinal surface. Intestinal bacteria and their components (pathogen-associated molecular patterns (PAMPs)) translocate into the lamina propria and are recognized by antigen-presenting cells such as dendritic cells (DCs), leading to activation of effector cells and aggravation of graft-versus-host disease (GVHD). B cells, Paneth cells and the mucous layer are known to be targets of GVHD, and damage to these creates a vicious circle exacerbating gut inflammation and bacterial translocation and resulting in worse survival after allo-HSCT. IEC, intestinal epithelial cell; NKT, natural killer T; TH17, T helper 17; Treg, regulatory T.