Figure 3.
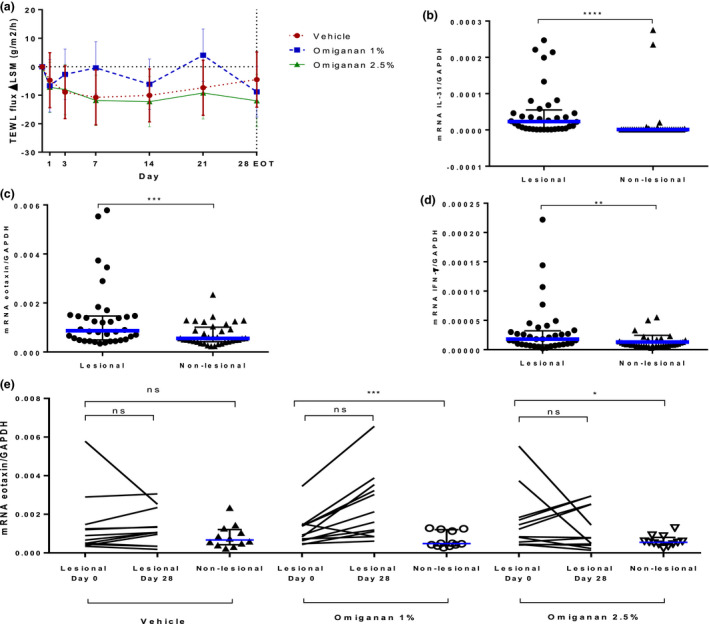
Pharmacodynamic effects of topical omiganan in the omiganan 1%, omiganan 2.5%, and vehicle gel groups. (a) Transepidermal water loss (TEWL) over time is depicted, showing improvement in the treatment groups and the vehicle group. Relative mRNA expressions in skin punch biopsy of markers (b) interleukin‐31, (c) eotaxin, and (d) interferon‐γ in lesional vs. nonlesional skin in mild to moderate atopic dermatitis patients at baseline. Data presented as median with interquartile range. Statistical significance is as follows: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, based on a paired t‐test on log‐transformed data. (e) Relative mRNA expression of eotaxin per treatment group before treatment of lesional skin (day 0) and after end of treatment of lesional skin (day 28) and nonlesional (NL) skin. No treatment effect could be seen with this marker.
