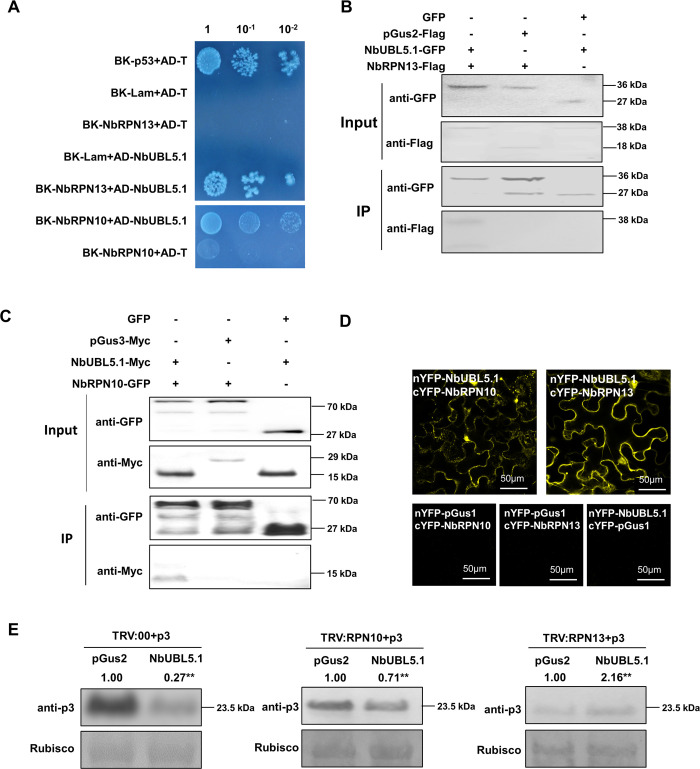
Fig 5. NbUBL5.1 interacts with the ubiquitin receptors in the 26S proteasome, NbRPN10 and NbRPN13, and their silencing inhibits NbUBL5.1-mediated p3 degradation.
A. Y2H showing the interaction of NbUBL5.1 with NbRPN10 and NbRPN13. B and C Co-IP showing the interaction of NbUBL5.1 with NbRPN10 and NbRPN13. pGus3 (The N-terminal peptide of Gus with 199 amino acids) was used as control. D. BiFC showing the interaction of NbUBL5.1 with NbRPN10 and NbRPN13. E. On NbRPN10- and NbRPN13-silenced plants, a reduction of p3-GFP protein caused by NbUBL5.1-Myc was detected. Results indicate that their silencing inhibits NbUBL5.1-mediated p3 degradation. On non-silenced plants, reduction of p3-GFP protein caused by NbUBL5.1-Myc was used as control. GFP and Myc-fused proteins were detected by western blot. Results are from three biological replicates. Band intensity in blots was calculated by ImageJ based on three replicates. A two-sample unequal variance directional t test was used to test the significance of the difference (**, p value<0.01).