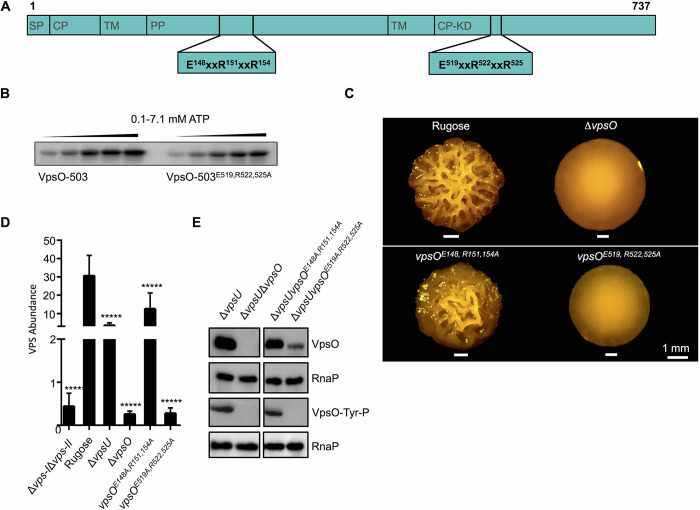
Fig 8. The ExxRxxR motifs alter VPS production in vivo and the VpsO oligomerization state without affecting kinase activity in vitro.
(A) Diagram of the VpsO protein highlighting the positions of the ExxRxxR motifs. (B) 50 μM VpsO-503 and VpsO-503E519A, R522A, R525A incubated with increasing concentrations of [γ-32P]-ATP were analyzed for autophosphorylation by gel electrophoresis; n≥2. (C) Colony corrugation phenotypes of the ExxRxxR motif mutants compared to the rugose strain after 5 days of growth at 25°C. (D) Quantification of VPS production by the periplasmic and ExxRxxR motif mutants compared to the rugose strain; n = 2 for biological replicates each with n = 3 for technical replicates. *****, p < 0.0005 by one-way Anova multiple comparisons test. The Δvps-IΔvps-II, rugose, ΔvpsU, and ΔvpsO control are identical to Fig 5C. (E) Western blot analysis of VpsO abundance and tyrosine phosphorylation; n≥3 The ΔvpsU and ΔvpsUΔvpsO controls are identical to Fig 7D.