Summary
Previous studies reported on the safety and applicability of mesenchymal stem/stromal cells (MSCs) to ameliorate pulmonary inflammation in acute respiratory distress syndrome (ARDS). Thus, multiple clinical trials assessing the potential of MSCs for COVID-19 treatment are underway. Yet, as SARS-inducing coronaviruses infect stem/progenitor cells, it is unclear whether MSCs could be infected by SARS-CoV-2 upon transplantation to COVID-19 patients. We found that MSCs from bone marrow, amniotic fluid, and adipose tissue carry angiotensin-converting enzyme 2 and transmembrane protease serine subtype 2 at low levels on the cell surface under steady-state and inflammatory conditions. We did not observe SARS-CoV-2 infection or replication in MSCs at steady state under inflammatory conditions, or in direct contact with SARS-CoV-2-infected Caco-2 cells. Further, indoleamine 2,3-dioxygenase 1 production in MSCs was not impaired in the presence of SARS-CoV-2. We show that MSCs are resistant to SARS-CoV-2 infection and retain their immunomodulation potential, supporting their potential applicability for COVID-19 treatment.
Keywords: SARS-CoV-2, COVID-19, mesenchymal stromal cells, inflammation, ARDS, stem cells
Highlights
-
•
MSCs carry ACE2 and TMPRSS2 only at very low levels on the cell surface
-
•
Inflammatory conditions do not change ACE2 and TMPRSS2 expression on MSCs
-
•
MSCs are resistant to SARS-CoV-2 infection
-
•
MSCs retain their immunomodulation potential in the presence of SARS-CoV-2
Schäfer and colleagues found that human MSCs carry ACE 2 and TMPRSS2 at very low levels on the cell surface under steady-state and inflammatory conditions. Using their in vitro model for infection with SARS-CoV-2 from clinical isolates they show that MSC are resistant to SARS-CoV-2 infection and retain their immunomodulation potential supporting their potential applicability for COVID-19 treatment.
Introduction
The current coronavirus disease 2019 (COVID-19) pandemic is posing substantial challenges to various medical disciplines, particularly to intensive care units in which patients with respiratory insufficiency are being treated (Phua et al., 2020).
After SARS-CoV-2 transmission, mainly via respiratory droplets, symptomatic patients show breathing difficulties and about 14% of the patients develop severe COVID-19, and 5% of the patients, mainly in the context of pre-existing conditions, such as cardiovascular disease, diabetes, or chronic respiratory disease, are at higher risk of a lethal course of the disease; a case-fatality rate of 49% in those who develop respiratory failure, septic shock, and/or multiple organ dysfunction (Tay et al., 2020; Wu and McGoogan, 2020). Specifically with regard to the lung, the leading pathophysiology of COVID-19 is a severe acute respiratory distress syndrome (ARDS), and respiratory failure due to ARDS is the main cause (70%) of death (Tay et al., 2020).
Inside the airways, SARS-CoV-2 targets epithelial cells and, further invasively, vascular endothelial cells and pulmonary macrophages (Tay et al., 2020). To date, two key factors for successful virus entry into the host cells have been identified, i.e., angiotensin-converting enzyme 2 (ACE2) being expressed on the surface of these cell types and acting as receptor for the S1 subunit of the viral spike protein, and the cellular serine protease TMPRSS2 for the necessary viral S protein priming (Hoffmann et al., 2020; Tay et al., 2020). The subsequent active virus replication and its release damage the infected host cell by inducing pyroptosis which, in turn, triggers the release of pro-inflammatory cytokines and chemokines, such as interleukin-6 (IL-6), IL-1β, interferon γ (IFN-γ), IFN-γ-induced protein 10, macrophage inflammatory protein 1α (MIP1α), MIP1β, and MCP1 (Tay et al., 2020). At this stage, attracted and activated monocytes, macrophages, and T cells not only maintain an inflammatory milieu but, by additionally releasing IFN-γ, create a pro-inflammatory feedback loop that further damages the lung tissue and induces capillary leakage (Tay et al., 2020). A sepsis-like perpetuation of the inflammatory response can create a generalized cytokine storm that eventually leads to multi-organ failure (Tay et al., 2020). The clinical relevance of the unbalanced inflammatory response is highlighted by the observation that the cytokine storm and sepsis symptoms are prominent (28%) causes of fatality in COVID-19 cases (Tay et al., 2020). Thus, controlling the disruptive inflammatory responses may be considered as a substantial component for therapeutic strategies for COVID-19.
Mesenchymal stem/stromal cells (MSCs) are potent immune regulators secreting immunomodulatory factors and interacting with a variety of immune cell types, such as T cells, B cells, dendritic cells, and macrophages (Fontaine et al., 2016). To date, the major sources for manufacture of MSC therapeutics are bone marrow (BM) and adipose tissue (Schäfer et al., 2016). Clinical applications of MSCs proved efficacy for immunopathologies, such as graft-versus-host disease (GvHD), organ graft rejection, as well as for autoimmune diseases (Schäfer, 2019). Previous preclinical and clinical studies showed the safety and applicability of MSC therapies to ameliorate the pulmonary inflammation in the context of ARDS. In addition to their immunomodulation potential, currently discussed mechanisms of how MSCs exert their beneficial effects in ARDS pathology include preservation of the epithelial and endothelial barrier, reduced impairment of alveolar fluid clearance, as well as possible antimicrobial activity (Chan et al., 2016; Walter et al., 2014).
Specifically, intravenously applied BM-MSCs reduced static lung elasticity, interstitial edema, and collagen fiber content in a murine ARDS model (Silva et al., 2019), and human umbilical cord-derived MSCs reduced the mortality in a rat ARDS model (Lee et al., 2017). Moreover, intravenous single-dose infusions of allogeneic, BM-derived human MSCs were well tolerated in patients with moderate to severe ARDS, as recently assessed in a multi-center study (Matthay et al., 2019; Wilson et al., 2015). Thus, multiple clinical trials evaluating the potential of MSCs for COVID-19 treatment are currently underway.
Yet, SARS-CoV were shown to infect and replicate in ACE2-expressing pulmonary progenitor cells, eventually killing them (Ling et al., 2006), and it is unclear if MSCs could be infected by SARS-CoV-2 upon transplantation in COVID-19 patients. Recently, we established an in vitro model for infection with SARS-CoV-2 from clinical isolates (Bojkova et al., 2020). In this model we tested the potential of SARS-CoV-2 to infect human MSCs from different sources and to replicate within these cells.
Results
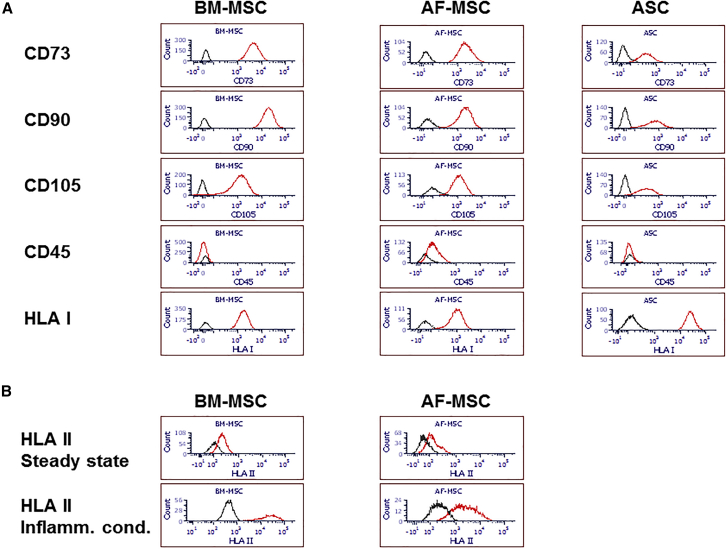
First, we confirmed the typical surface characteristics of the MSCs from the three sources: BM, amniotic fluid (AF), and adipose tissue. BM-MSCs, AF-MSCs, and adipose stem cells (ASCs) showed positive marker expression, such as CD73, CD90, and CD105, and lacked CD45 on the cell surface (Figure 1A). As expected, under steady-state conditions the human leucocyte antigen (HLA)-DR expression on BM-MSCs and AF-MSCs was very low, but was strongly increased upon exposure to inflammatory conditions, validating the in vitro inflammation model for the MSCs (Figure 1B).
Figure 1.
Human MSC Characterization
Human MSC characterization by flow cytometry under steady-state conditions (A) and inflammatory conditions (B). Histograms show fluorescence of specific antibodies (red lines) compared with unstained cells (black lines).
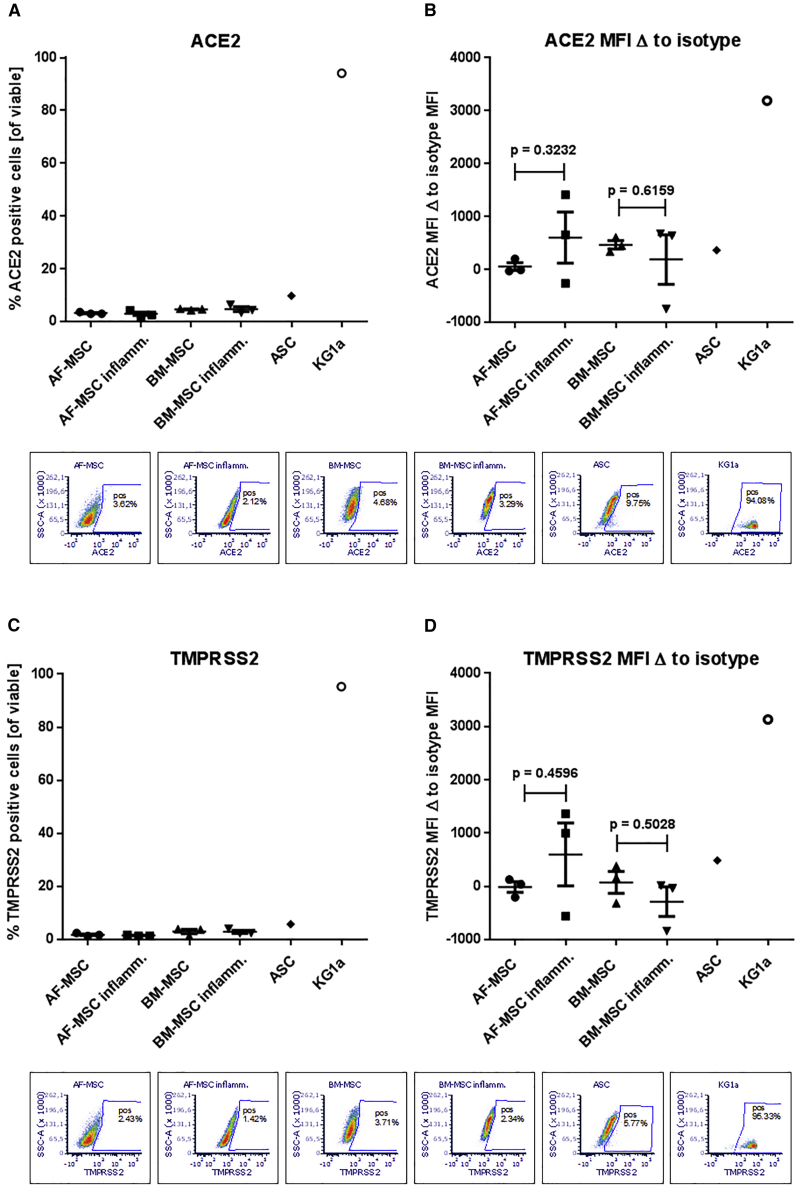
Next, we investigated the presence of both, to date identified, key entry factors for SARS-CoV-2, i.e., angiotensin-converting enzyme 2 (ACE2) and TMPRSS2, on the MSC surface. As ACE2 is an interferon-stimulated gene (Ziegler et al., 2020), and to model the inflammatory environment in COVID-19, we tested the ACE2 and TMPRSS2 expression also under inflammatory conditions in our MSC-peripheral blood mononuclear cell (PBMNC) co-culture system. In contrast to KG1a control cells both ACE2 and TMPRSS2 proteins could be detected at very low levels on BM-MSCs, AF-MSCs, and ASCs with similar percentages and mean fluorescence intensities (Figure 2). Exposure to inflammatory conditions did not increase the low expression of ACE2 or TMPRSS2 on the surface of BM-MSCs and AF-MSCs (Figure 2).
Figure 2.
ACE2 and TMPRSS2 Expression on Human MSCs
ACE2 (A and B) and TMPRSS2 (C and D) expression assessed by flow cytometry. On the controls (KG1a cells), ACE2 and TMPRSS2 were highly expressed, whereas very low ACE2 and TMPRSS2 expression was detected on the surface of MSCs; two-tailed paired t test; data presented as means of three donors (BM-MSCs, AF-MSCs), or one donor/cell line (ASCs, KG1a), respectively; error bars, SEM; dot plots show viable cells with gate set according to isotype control for the respective conditions.
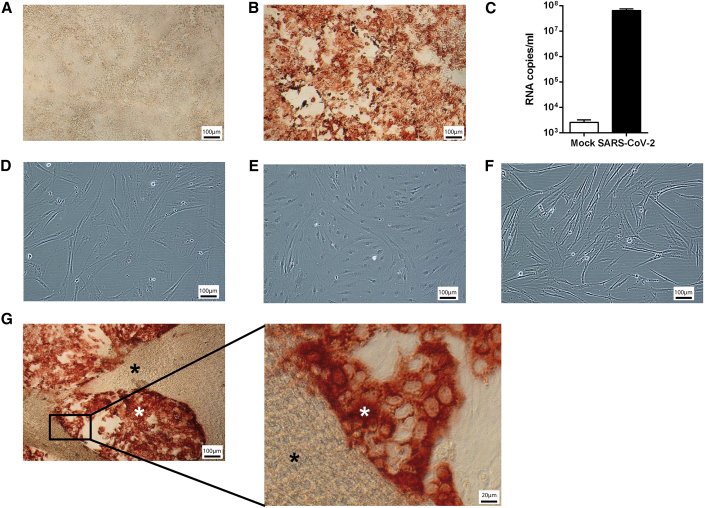
Next, we tested the potential of SARS-CoV-2 to infect MSCs under different conditions. Control Caco-2 cells showed high SARS-CoV-2 infection rates with massive intracellular S protein accumulation indicating virus entry, and analysis by qPCR confirmed SARS-CoV-2 replication in Caco-2 cells (Figures 3A–3C). In contrast, neither BM-MSCs nor ASCs could be infected with SARS-CoV-2 (Figures 3C and 3D). Moreover, BM-MSCs remained resistant to SARS-CoV-2 infection under inflammatory conditions and even at close contact to highly infected Caco-2 cells (Figures 3E and 3F).
Figure 3.
Evaluation of SARS-CoV-2 Infection of MSCs
Evaluation of SARS-CoV-2 infection of MSCs under steady-state and inflammatory conditions and in the presence of SARS-CoV-2-infected Caco-2 cells. SARS-CoV-2 infection is identified by SARS-CoV-2 S protein staining (red).
All MSCs and Caco-2 cells experiments were repeated in three independent settings from three BM-MSC donors and three ASC donors, and were performed in three biological replicates each. One representative picture is shown for each condition. (A) Caco-2 cells without SARS-CoV-2; (B) Caco-2 cells with SARS-CoV-2 MOI1; (C) SARS-CoV-2 replication quantified by qPCR detecting high copy numbers in Caco-2 cells infected by SARS-CoV-2; error bars: SD; (D) BM-MSC steady state with SARS-CoV-2 MOI1; (E) ASC steady state with SARS-CoV-2 MOI1; (F) BM-MSC inflammatory conditions with SARS-CoV-2 MOI1; (G) Co-culture BM-MSC:Caco-2 cells (10:1) with SARS-CoV-2 MOI1; BM-MSCs (black star) + Caco-2 cells (white star). Scale bars, 100 μm and 20 μm (inset in G).
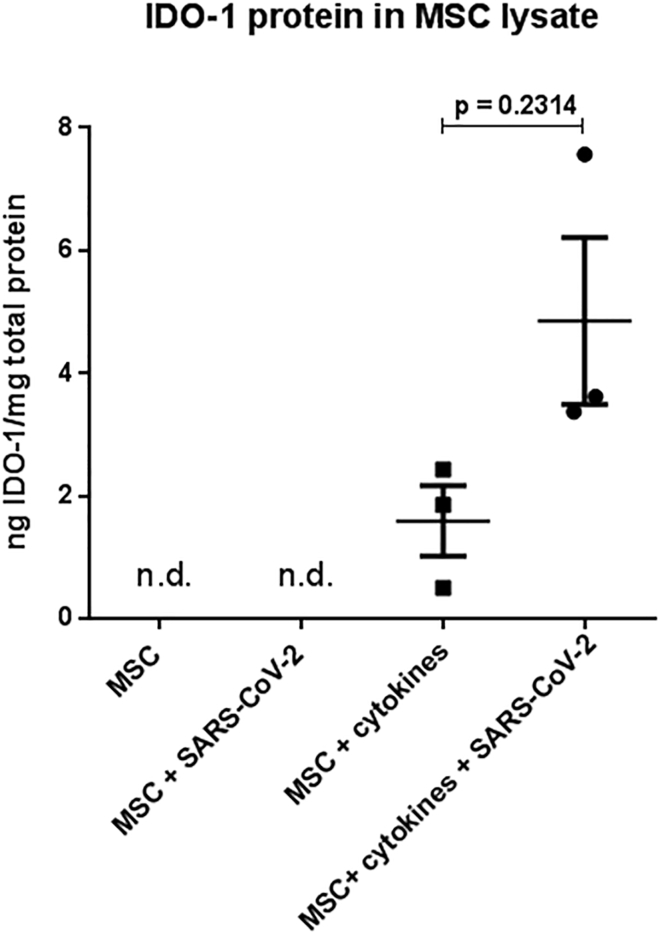
We further investigated if, even when not being infected, the presence of SARS-CoV-2 would affect the indoleamine 2,3-dioxygenase 1 (IDO-1) production in MSCs, which is the main surrogate factor for the immunomodulation capacity of MSCs. The production of IDO-1 is triggered by pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), IL-1β, and IFN-γ1b. Indeed, we did not detect IDO-1 protein in BM-MSC lysates under steady-state conditions or in the presence of SARS-CoV-2 without cytokines. In contrast, and as expected, the BM-MSCs produced IDO-1 after cytokine exposure. Notably, we did not observe an impairment of MSC IDO-1 production after cytokine exposure in the presence of SARS-CoV-2 (Figure 4).
Figure 4.
Evaluation of IDO-1 Production by MSCs
BM-MSC lysates analyzed by IDO-1 ELISA. Two-tailed paired t test; N = 3 biological replicates (three individual BM-MSC donors); data are presented as means of three technical replicates per donor; error bars, SEM; n.d., not detectable.
Discussion
Severe courses of COVID-19 are characterized by ARDS together with cytokine storm-mediated multi-organ failure, and to date vaccines are still under development. Consequently, therapeutic approaches include the evaluation of immunosuppressive drugs. Recently released intermediate data from the RECOVERY trial (NCT04381936) showed a reduced mortality of about 20% in patients on ventilators who received dexamethasone (https://clinicaltrials.gov/ct2/show/NCT04381936 RECOVERY, 2020).
In line with this, MSCs, being proven as clinically potent immunomodulating cell therapeutics, as shown for GvHD (Voermans and Hazenberg, 2020), are currently being evaluated for their applicability to treat COVID-19 with, to date, more than 40 registered clinical MSC trials at ClinicalTrials.gov. Moreover, first clinical data suggested that MSC application to patients suffering with non-COVID ARDS were safe (Matthay et al., 2019). Yet, SARS-inducing corona viruses were shown to infect progenitor cells (Ling et al., 2006), and thus it is reasonable to investigate the possibility of SARS-CoV-2 infection of MSCs and the potential implications. On the one hand, SARS-CoV-2 infection of MSCs could lead to rapid clearance of the transplanted MSCs thus hampering their therapeutic efficacy. On the other hand, SARS-CoV-2 could induce apoptosis of MSCs that could further exert desired immunomodulation effects such as induction of IDO-1 production, as shown previously for GvHD (Galleu et al., 2017). In addition, SARS-CoV-2-infected MSCs might provoke unknown, and thus uncontrollable, side effects, such as aggravation of COVID-19 symptoms, complications, or transformation (Cason et al., 2017; Oberstein and Shenk, 2017).
To date the only available data addressing the question of whether or not human MSCs would carry ACE2 and/or TMPRSS2 was reported in a recent study on seven patients who received allogeneic MSCs of undisclosed source for the treatment of COVID-19. Here, RNA sequencing (RNA-seq) analysis showed low expression of both ACE2 and TMPRSS2 gene transcripts (Leng et al., 2020). In a hallmark paper Ziegler et al. (2020) screened multiple human and non-human tissues using single-cell RNA-seq and found ACE2 and TMPRSS2 genes co-expressing type II pneumocytes, ileal absorptive enterocytes, and nasal goblet secretory cells, but this dataset did not include human BM, AF, adipose tissue, or ex-vivo-cultured MSCs, a prerequisite for their clinical use. Moreover, substantial discrepancies of up to 40% between transcript and protein are common due to post-transcriptional regulation or protein degradation (Kuci et al., 2019; Schwanhausser et al., 2011).
Therefore, we analyzed the expression of both key entry factors on the surface of human MSCs from sources that have been mainly used for clinical MSC therapeutic manufacture, i.e., BM-MSCs and ASCs (Schäfer et al., 2016), as well as MSCs isolated from AF, another promising source (Moraghebi et al., 2017). Confirming the above-mentioned transcript data on protein level we did not detect noteworthy numbers of ACE2+ or TMPRSS2+ cells within the BM-MSC and AF-MSC preparations, and only very few (<10%) ACE2+ or TMPRSS2+ cells among the ASCs. Under inflammatory conditions we observed the expected upregulation of HLA-DR on the MSC surface, but we did not detect changes of ACE2 or TMPRSS2 expression. This is of relevance, as ACE2 gene expression is upregulated by IFN-α2 and IFN-γ, yet, as it appears to date, in a cell-type-specific manner, i.e., mainly affecting epithelial cells (Ziegler et al., 2020). However, pathways independent of IFNs that regulate ACE2 or TMPRSS2 expression may exist, and it is not clear if the cell populations with IFN-dependent ACE2 regulation are actually sensitive for SARS-CoV-2 infection (Su and Jiang, 2020), or if ACE2 and TMPRSS2 are the exclusive ports of entry for non-epithelial cells. The roles of cytokines and IFNs in COVID-19 pathogenesis and for the development of therapeutic options are still under debate (Chu et al., 2020; Su and Jiang, 2020), whereas it is clear that exposure to (pulmonary) inflammatory conditions substantially impacts the gene expression of MSCs as well as the above-mentioned change of HLA-DR expression (Abreu et al., 2019; Andrzejewska et al., 2019). Therefore, we used our allogeneic co-culture system to model the inflammatory environment for the MSCs, i.e., the MSCs share the medium with pooled allogeneic PBMNCs that are additionally stimulated with phytohemagglutinin (PHA), thus exposing the MSCs to the complete repertoire of the cytokines and IFNs being secreted by the PBMNCs.
We used our in vitro SARS-CoV-2 infection model (Bojkova et al., 2020) to evaluate the potential of SARS-CoV-2 to infect human BM-MSCs and ASCs. Specifically, we tested three conditions: (1) steady-state conditions, i.e., without additional stimuli, (2) inflammatory conditions as described above, and (3) co-culture with infected Caco-2 cells. The BM-MSCs and ASCs could not be infected with SARS-CoV-2 in the steady state, and the further tested BM-MSCs remained resistant under inflammatory conditions as well as in co-culture with SARS-CoV-2-infected Caco-2 cells. We aimed to model the local inflammatory environment and the generalized cytokine storm in COVID-19. Moreover, we analyzed the IDO-1 production of MSCs, which is an accepted surrogate for their immunomodulation potential (Galleu et al., 2017). Thus, the observed resistance of MSCs to SARS-CoV-2 infection in condition (2), as well as their unimpaired IDO-1 production in the presence of SARS-CoV-2 and cytokines, suggest that clinically applied MSCs would deliver their expected immunomodulation function in COVID-19 as they did in various preclinical ARDS models (Johnson et al., 2017).
Under steady-state conditions, as well as under inflammatory conditions, we observed very low expression of the current known entry molecules, ACE2 and TMPRSS2, on the MSC surface, as well as resistance of MSCs to SARS-CoV-2 infection. Both observations likely confirm ACE2 and TMPRSS2 as relevant factors for SARS-CoV-2 infection, not only for epithelial cells but also for mesenchymal cells. However, we cannot exclude alternative routes of SARS-CoV-2 entry, particularly for non-epithelial cells.
It is reasonable to hypothesize that the transplanted MSCs may interact with at least some of the various cell types in the lung tissue from the endothelial side via the stroma to the epithelial side. The results obtained from the MSC-Caco-2 co-culture system in the presence of SARS-CoV-2 (condition (3)) showed that even the direct contact with infected Caco-2 cells with massive virus replication did not lead to MSC infection with SARS-CoV-2. This sheds an interesting light on possible interactions of infected cells with MSCs, yet more detailed investigations on possible interactions of MSCs with pulmonary cells are warranted.
After systemic administration, MSCs were shown to accumulate in the lung and as such could be augmented by pulmonary inflammation with local deposition of factors acting as chemoattractants for MSCs (Krueger et al., 2018). This might lead to side effects, such as pulmonary artery thrombosis, but reports of the hemodynamically relevant impact of MSC entrapment in the lung are inconsistent (Nystedt et al., 2013). Moreover, MSCs were reported to express tissue factors and as such they were reported to have pro-coagulant activity (Christy et al., 2017), an issue that needs further investigations particularly in the context of the relevant involvement of coagulation in COVID-19 pathogenesis (Jose and Manuel, 2020).
Altogether, we show that clinically relevant MSC entities are resistant to SARS-CoV-2 infection under conditions that are pathophysiological relevant for COVID-19. Moreover, our data suggest that MSCs keep their immunomodulation potential in the presence of SARS-CoV-2, thus supporting their ongoing clinical evaluation for the treatment of COVID-19. However, further investigations are warranted to better understand the possible impact of MSC interactions with different cell types and their systemic effects, including possible pro-coagulant activity in the presence of SARS-CoV-2 or in COVID-19.
Experimental Procedures
MSC Isolation and Cultivation
Human BM samples were obtained from three healthy donors undergoing BM donation after informed consent and ethical committee approval. Trapped cells were removed from the three-way filter system used to process the BM aspirate by retrograde flushing with PBS (Thermo Fisher Scientific, Waltham, MA, USA), and BM mononuclear cells (MNCs) were isolated by density gradient centrifugation on lymphocyte separation medium (Lonza, Basel, Switzerland). The BM-MSC isolation was performed as described previously (Siegel et al., 2013). In brief, the MNCs were re-suspended in standard cell culture medium, composed of Alpha MEM (Lonza), 10% human platelet lysate (hPL) (manufactured in-house), 2 IU/mL heparin (Ratiopharm, Ulm, Germany), and 1% penicillin-streptomycin (Thermo Fisher Scientific) and seeded into cell culture flasks at a density of 1.0 × 105 cells/cm2. Non-adherent cells were washed away after 24 h and the persistent cells were maintained in cell culture medium at 37°C in a humidified atmosphere with 5% CO2. When MSCs reached subconfluency, they were detached using 0.05% Trypsin-EDTA (Thermo Fisher Scientific) and either cryopreserved or seeded at a density of 1,000 cells/cm2.
Human adipose-derived adult mesenchymal stromal cells (ASCs) were isolated from lipoaspirates from three donors undergoing cosmetic liposuction in accordance with the local ethical committee and isolated as described previously (Baer et al., 2013). In brief, Dulbecco's modified Eagle's medium (Sigma, Taufkirchen, Germany) was used with a physiologic glucose concentration (100 mg/dL) supplemented with 10% fetal bovine serum (FBS) (PAA, Cölbe, Germany) as the culture medium. The medium was replaced every 3 days. Subconfluent cells (85%–90% confluency) were passaged by trypsinization.
For the isolation of AF-MSCs, AF was collected by amniocentesis from three patients with polyhydramnios after informed consent and ethical committee approval. The AF-MSC isolation was performed with modifications as described previously (De Coppi et al., 2007). In brief, after centrifugation of the AF the cells were re-suspended in standard cell culture medium, composed of Alpha MEM (Lonza), 15% ES-FBS (Thermo Fisher Scientific), 18% Chang B medium (Irvine Scientific, Santa Ana, CA, USA), 2% Chang C medium supplement (Irvine Scientific), 1% glutamine (Thermo Fisher Scientific), and 1% penicillin-streptomycin (Thermo Fisher Scientific) and seeded into cell culture flasks at a density of 1.6–3.6 × 105 cells/cm2. Non-adherent cells were washed away after 24 h and the persistent cells were maintained in cell culture medium at 37°C in humidified atmosphere with 5% CO2. When AF-MSCs reached subconfluency, they were detached using 0.05% Trypsin-EDTA (Thermo Fisher Scientific) and either cryopreserved or seeded at a density of 1,000 cells/cm2.
For preparation of infection experiments, cryopreserved MSC passage (P) 0 were thawed and seeded at a density of 2,000 cells/cm2 in modified cell culture medium without heparin and replacement of hPL by FBS (Sigma-Aldrich, St. Louis, MO, USA).
SARS-CoV-2 Infection and Immunostaining
The Caco-2 cell line was obtained from DSMZ (Braunschweig, Germany). The cells were grown at 37°C in minimal essential medium supplemented with 10% FBS, 100 IU/mL penicillin, and 100 μg/mL of streptomycin. All culture reagents were purchased from Sigma (Munich, Germany). Cells were authenticated by short tandem repeat analysis and tested for mycoplasma contamination. The isolate SARS-CoV-2/1/Human/2020/Frankfurt (Hoehl et al., 2020) was cultivated in Caco-2 cells as previously described for SARS-CoV strain FFM-1 523 (Cinatl et al., 2004). Virus titers were determined as half maximal tissue culture infective dose (TCID50)/mL in confluent cells in 96-well microtiter plates (Cinatl et al., 2003, 2004). MSCs were incubated with virus at MOI1 for 2 h. After this period, cells were washed, supplemented with fresh medium, and cultured for 5 days. Immunostaining of SARS-CoV-2 was performed as described previously (Bojkova et al., 2020). Cells were fixed with acetone/methanol (40:60) solution and incubated with primary antibody anti-spike (1:1,500, Sino Biological, catalog no. 40150-R007, Singapore) which was detected with a peroxidase-conjugated anti-rabbit secondary antibody (1:1,000, Dianova, catalog no. SKU:111-035-045), followed by addition of AEC substrate. All work with infectious viruses was performed in a biosafety level 3 facility.
Quantification of SARS-CoV-2 RNA
RNA from cell culture supernatant was isolated with the QIAamp Viral RNA Kit (QIAGEN) according to the manufacturer's instructions. SARS-CoV-2 RNA was detected by One-Step qRT-PCR analysis using the Luna Universal One-Step RT-qPCR Kit (New England Biolabs) and primers directed for RNA-dependent RNA polymerase (RdRp): SARSr-F2 (GTG ARA TGG TCA TGT GTG GCG G) and RdRP_SARSr-R1 (CAR ATG TTA AAS ACA CTA TTA GCA TA). Standard curves were created using plasmid DNA (pEX-A128-RdRP) harboring the corresponding amplicon regions for the RdRP target sequence according to GenBank accession no. NC_045512. The amount of RNA was calculated as RNA copies/mL.
Flow Cytometry
MSCs were analyzed at P1 by flow cytometry (LSRFortessa; Becton Dickinson, Heidelberg, Germany) with regard to the ISCT MSC criteria. Cells were stained with 7-AAD viability dye (BioLegend, San Diego, CA, USA) and antibodies against CD45 (catalog no. 560777), CD73 (catalog no. 563199), CD90 (catalog no. 561558), CD105 (catalog no. 562380), HLA-ABC (catalog no. 555555), or HLA-DR (catalog no. 562804) (all from BD Biosciences unless otherwise noted).
ASCs (P1) were analyzed by flow cytometry under steady state, BM-MSCs (P2) and AF-MSCs (P4) under steady-state and inflammatory conditions for expression of ACE2 and TMPRSS2, respectively. Cells were stained with 7-AAD viability dye and antibodies against ACE2 (catalog no. sc-390851) and TMPRSS2 (catalog no. sc-515727) (Santa Cruz Biotechnology, Dallas, TX, USA), IgG1 kappa light chain (catalog no. 555749, BD Biosciences) served as isotype control, and the cell line KG1a as positive control.
Flow cytometry data were analyzed using FCS Express 6 Flow Software (De Novo Software, Pasadena, CA, USA).
Inflammatory Condition
BM-MSCs were seeded at a density of 96,000 cells per well into 6-well plates and allowed to adhere overnight. For indirect co-culturing permeable cell culture inserts with 0.4-μm pores (Greiner Bio-One, Frickenhausen, Germany) were placed into the 6-well plates. PBMNCs (derived from buffy coats from five healthy donors, pooled, and cryopreserved) were seeded at a density of 1 × 106 cells per insert, stimulated with 10 μg/mL PHA (Sigma-Aldrich), and co-cultured for 72 h. Effective inflammation was monitored by flow cytometric detection of upregulation of HLA-DR.
Quantification of IDO-1 Production
Production of IDO-1 was stimulated in BM-MSCs with or without exposure to SARS-CoV-2 by TNF-α, IFN-γ1b (Miltenyi Biotec, Bergisch Gladbach, Germany), and IL-1β (PeproTech, Hamburg, Germany), each 20 ng/mL, for 48 h. BM-MSCs exposed to SARS-CoV-2 without cytokine stimulation were analyzed as well. MSCs grown in medium containing 10% FBS were stimulated as well and served as positive controls.
Subsequently, the MSCs were harvested, lysed, and analyzed in triplicate with an ELISA specific for IDO-1 (Cloud-Clone, Katy, TX, USA).
Statistical Analyses
Quantitative data are presented as means ± standard error of the means (SEM), or as means ± standard deviation (SD). For comparison of selected datasets the two-tailed t test was used (Figures 2 and 4) using GraphPad Prism (La Jolla, CA, USA). Exact p values are reported in the figures, and p values of <0.05 were considered statistically significant.
Author Contributions
R.S. and J.C. designed the research, analyzed the data, and wrote the manuscript. G.S., M.B., and D.B. performed the experiments, analyzed the data, and reviewed the manuscript. P.C.B. and S.K. provided human ASCs, anti-ACE2, and anti-TMPRSS2 antibodies, and reviewed the manuscript. E.S. and S.C. discussed the results and reviewed the manuscript.
Acknowledgments
The authors wish to thank Kerstin Euler and Lena Stegmann for technical assistance and the Johanna-Quandt-Stiftung for financial support.
Published: October 8, 2020
References
- Abreu S.C., Rolandsson E.S., Dearborn J., Goodwin M., Coffey A., Borg Z.D., Dos Santos C.C., Wargo M.J., Cruz F.F., Loi R. Lung inflammatory environments differentially alter mesenchymal stromal cell behavior. Am. J. Physiol. Lung Cell Mol. Physiol. 2019;317:L823–L831. doi: 10.1152/ajplung.00263.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrzejewska A., Catar R., Schoon J., Qazi T.H., Sass F.A., Jacobi D., Blankenstein A., Reinke S., Kruger D., Streitz M. Multi-parameter analysis of biobanked human bone marrow stromal cells shows little influence for donor age and mild comorbidities on phenotypic and functional properties. Front. Immunol. 2019;10:2474. doi: 10.3389/fimmu.2019.02474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer P.C., Kuci S., Krause M., Kuci Z., Zielen S., Geiger H., Bader P., Schubert R. Comprehensive phenotypic characterization of human adipose-derived stromal/stem cells and their subsets by a high throughput technology. Stem Cells Dev. 2013;22:330–339. doi: 10.1089/scd.2012.0346. [DOI] [PubMed] [Google Scholar]
- Bojkova D., Klann K., Koch B., Widera M., Krause D., Ciesek S., Cinatl J., Munch C. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature. 2020;583:469–472. doi: 10.1038/s41586-020-2332-7. [DOI] [PubMed] [Google Scholar]
- Cason C., Campisciano G., Zanotta N., Valencic E., Delbue S., Bella R., Comar M. SV40 infection of mesenchymal stromal cells from Wharton's jelly drives the production of inflammatory and tumoral mediators. J. Cell Physiol. 2017;232:3060–3066. doi: 10.1002/jcp.25723. [DOI] [PubMed] [Google Scholar]
- Chan M.C., Kuok D.I., Leung C.Y., Hui K.P., Valkenburg S.A., Lau E.H., Nicholls J.M., Fang X., Guan Y., Lee J.W. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc. Natl. Acad. Sci. U S A. 2016;113:3621–3626. doi: 10.1073/pnas.1601911113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy B.A., Herzig M.C., Montgomery R.K., Delavan C., Bynum J.A., Reddoch K.M., Cap A.P. Procoagulant activity of human mesenchymal stem cells. J. Trauma Acute. Care Surg. 2017;83:S164–S169. doi: 10.1097/TA.0000000000001485. [DOI] [PubMed] [Google Scholar]
- Chu H., Chan J.F., Wang Y., Yuen T.T., Chai Y., Hou Y., Shuai H., Yang D., Hu B., Huang X. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. 2020;71:1400–1409. doi: 10.1093/cid/ciaa410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinatl J., Jr., Hoever G., Morgenstern B., Preiser W., Vogel J.U., Hofmann W.K., Bauer G., Michaelis M., Rabenau H.F., Doerr H.W. Infection of cultured intestinal epithelial cells with severe acute respiratory syndrome coronavirus. Cell Mol. Life Sci. 2004;61:2100–2112. doi: 10.1007/s00018-004-4222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coppi P., Bartsch G., Jr., Siddiqui M.M., Xu T., Santos C.C., Perin L., Mostoslavsky G., Serre A.C., Snyder E.Y., Yoo J.J. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- Fontaine M.J., Shih H., Schafer R., Pittenger M.F. Unraveling the mesenchymal stromal cells' paracrine immunomodulatory effects. Transfus. Med. Rev. 2016;30:37–43. doi: 10.1016/j.tmrv.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Galleu A., Riffo-Vasquez Y., Trento C., Lomas C., Dolcetti L., Cheung T.S., von B.M., Barbieri L., Halai K., Ward S. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 2017;9:9eaam7828. doi: 10.1126/scitranslmed.aam7828. [DOI] [PubMed] [Google Scholar]
- Hoehl S., Rabenau H., Berger A., Kortenbusch M., Cinatl J., Bojkova D., Behrens P., Boddinghaus B., Gotsch U., Naujoks F. Evidence of SARS-CoV-2 infection in returning travelers from Wuhan, China. N. Engl. J. Med. 2020;382:1278–1280. doi: 10.1056/NEJMc2001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.L., Soeder Y., Dahlke M.H. Concise review: mesenchymal stromal cell-based approaches for the treatment of acute respiratory distress and sepsis syndromes. Stem Cells Transl. Med. 2017;6:1141–1151. doi: 10.1002/sctm.16-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir. Med. 2020;8:e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger T.E.G., Thorek D.L.J., Denmeade S.R., Isaacs J.T., Brennen W.N. Concise review: mesenchymal stem cell-based drug delivery: the good, the bad, the ugly, and the promise. Stem Cells Transl. Med. 2018;7:651–663. doi: 10.1002/sctm.18-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuci S., Kuci Z., Schafer R., Spohn G., Winter S., Schwab M., Salzmann-Manrique E., Klingebiel T., Bader P. Molecular signature of human bone marrow-derived mesenchymal stromal cell subsets. Sci. Rep. 2019;9:1774. doi: 10.1038/s41598-019-38517-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F.Y., Chen K.H., Wallace C.G., Sung P.H., Sheu J.J., Chung S.Y., Chen Y.L., Lu H.I., Ko S.F., Sun C.K. Xenogeneic human umbilical cord-derived mesenchymal stem cells reduce mortality in rats with acute respiratory distress syndrome complicated by sepsis. Oncotarget. 2017;8:45626–45642. doi: 10.18632/oncotarget.17320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., Shan G., Meng F., Du D., Wang S. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling T.Y., Kuo M.D., Li C.L., Yu A.L., Huang Y.H., Wu T.J., Lin Y.C., Chen S.H., Yu J. Identification of pulmonary Oct-4+ stem/progenitor cells and demonstration of their susceptibility to SARS coronavirus (SARS-CoV) infection in vitro. Proc. Natl. Acad. Sci. U S A. 2006;103:9530–9535. doi: 10.1073/pnas.0510232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay M.A., Calfee C.S., Zhuo H., Thompson B.T., Wilson J.G., Levitt J.E., Rogers A.J., Gotts J.E., Wiener-Kronish J.P., Bajwa E.K. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir. Med. 2019;7:154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraghebi R., Kirkeby A., Chaves P., Ronn R.E., Sitnicka E., Parmar M., Larsson M., Herbst A., Woods N.B. Term amniotic fluid: an unexploited reserve of mesenchymal stromal cells for reprogramming and potential cell therapy applications. Stem Cell Res. Ther. 2017;8:190. doi: 10.1186/s13287-017-0582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystedt J., Anderson H., Tikkanen J., Pietila M., Hirvonen T., Takalo R., Heiskanen A., Satomaa T., Natunen S., Lehtonen S. Cell surface structures influence lung clearance rate of systemically infused mesenchymal stromal cells. Stem Cells. 2013;31:317–326. doi: 10.1002/stem.1271. [DOI] [PubMed] [Google Scholar]
- Oberstein A., Shenk T. Cellular responses to human cytomegalovirus infection: induction of a mesenchymal-to-epithelial transition (MET) phenotype. Proc. Natl. Acad. Sci. U S A. 2017;114:E8244–E8253. doi: 10.1073/pnas.1710799114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phua J., Weng L., Ling L., Egi M., Lim C.M., Divatia J.V., Shrestha B.R., Arabi Y.M., Ng J., Gomersall C.D. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir. Med. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECOVERY . 2020. Randomised Evaluation of COVID-19 Therapy (RECOVERY)https://clinicaltrials.gov/ct2/show/NCT04381936 [Google Scholar]
- Schäfer R. Advanced cell therapeutics are changing the clinical landscape: will mesenchymal stromal cells be a part of it? BMC. Med. 2019;17:53. doi: 10.1186/s12916-019-1289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer R., Spohn G., Baer P.C. Mesenchymal stem/stromal cells in regenerative medicine: can preconditioning strategies improve therapeutic efficacy? Transfus. Med. Hemother. 2016;43:256–267. doi: 10.1159/000447458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanhausser B., Busse D., Li N., Dittmar G., Schuchhardt J., Wolf J., Chen W., Selbach M. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- Siegel G., Kluba T., Hermanutz-Klein U., Bieback K., Northoff H., Schafer R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC. Med. 2013;11:146. doi: 10.1186/1741-7015-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J.D., de Castro L.L., Braga C.L., Oliveira G.P., Trivelin S.A., Barbosa-Junior C.M., Morales M.M., Dos Santos C.C., Weiss D.J., Lopes-Pacheco M. Mesenchymal stromal cells are more effective than their extracellular vesicles at reducing lung injury regardless of acute respiratory distress syndrome etiology. Stem Cells Int. 2019;2019:8262849. doi: 10.1155/2019/8262849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Jiang S. A suspicious role of interferon in the pathogenesis of SARS-CoV-2 by enhancing expression of ACE2. Signal. Transduct. Target Ther. 2020;5:71. doi: 10.1038/s41392-020-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay M.Z., Poh C.M., Renia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voermans C., Hazenberg M.D. Cellular therapies for graft versus host disease: a tale of tissue repair and tolerance. Blood. 2020;136:410–417. doi: 10.1182/blood.2019000951. [DOI] [PubMed] [Google Scholar]
- Walter J., Ware L.B., Matthay M.A. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir. Med. 2014;2:1016–1026. doi: 10.1016/S2213-2600(14)70217-6. [DOI] [PubMed] [Google Scholar]
- Wilson J.G., Liu K.D., Zhuo H., Caballero L., McMillan M., Fang X., Cosgrove K., Vojnik R., Calfee C.S., Lee J.W. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir. Med. 2015;3:24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–1035. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]