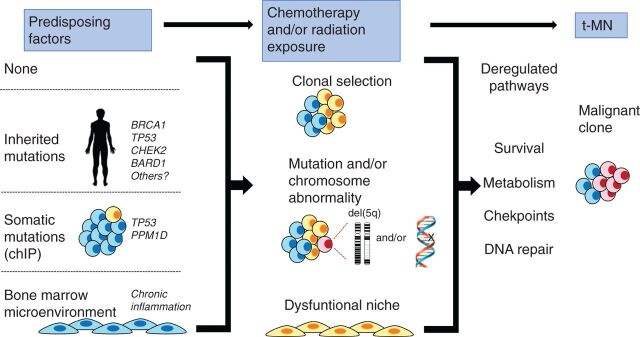
Figure 7.
Model illustrating how multiple, converging pathways may lead to t-MN. Left, various predisposing factors that may influence whether a patient develops t-MN. A patient may have no predisposing factors, have an inherited mutation (predisposing them to aberrant DNA repair), have a preexisting somatic mutation (CHIP), or have an aberrant BM microenvironment (due to aging and/or chronic inflammation). Middle, how multiple cycles of cytotoxic therapy may promote clonal expansion of cells with preexisting and/or newly acquired mutations and alter the BM microenvironment, possibly via therapy-induced senescence. Together, this creates permissive conditions for malignant transformation of hematopoietic cells. Right, how these changes converge to deregulate the DNA damage response and cell-cycle checkpoint, as well as enhance metabolism and survival, ultimately leading to t-MN.