Abstract
Background
While providers are challenged with treatment decisions during the coronavirus disease 2019 (COVID-19) crisis, decision making ultimately falls in the hands of patients—at present, their perspective is poorly understood.
Objective
To ascertain renal cell carcinoma (RCC) patients’ perspectives on COVID-19 and understand the associated implications for treatment.
Design, setting, and participants
An online survey of RCC patients was conducted from March 22 to March 25, 2020, disseminated through social media and patient networking platforms. The survey comprised 45 items, including baseline demographic, clinicopathologic, and treatment-related information. Patients were additionally queried regarding their anxiety level related to COVID-19 and associated implications for their cancer diagnosis.
Intervention
An online survey study.
Outcome measurements and statistical analysis
Descriptive statistics with graphical outputs were used to characterize survey results.
Results and limitations
A total of 539 patients (male:female 39%:58%) from 14 countries responded. Of them, 71% felt that their risk of COVID-19 infection was higher than the general population, and 27% contacted their physician to establish this. Among patients with localized disease (40%), most (42%) had scheduled surveillance scans within 6 wk–65% were unwilling to delay scans. Among patients with metastatic disease, 76% were receiving active therapy. While most patients preferred not to defer therapy (51%), patients receiving immune therapy regimens were less amenable to deferring therapy than those receiving targeted treatment (20% vs 47%).
Conclusions
Despite high levels of anxiety surrounding COVID-19, many patients with RCC were inclined to adhere to existing schedules of surveillance (localized disease) and systemic treatment (metastatic disease).
Patient summary
The coronavirus disease 2019 (COVID-19) pandemic has prompted many doctors to develop different treatment strategies for cancer and other chronic conditions. Given the importance of the patient voice in these strategies, we conducted a survey of patients with kidney cancer to determine their treatment preferences. Our survey highlighted that most patients prefer to continue their current strategies of kidney cancer treatment and monitoring.
Keywords: Renal cell carcinoma, Health care survey, Frustration, Qualitative study
Take Home Message
Through an online survey of 539 renal cell carcinoma patients conducted over a 3-d span, we identified that patients have significant anxiety related to coronavirus disease 2019 (COVID-19), which is counterbalanced by a similar level of anxiety related to cancer recurrence or progression. The majority of patients did not want to alter previously designated plans for cancer surveillance or treatment.
1. Introduction
The coronavirus disease 2019 (COVID-19) crisis is placing immense strain on health care systems worldwide. Data from initial studies suggest that patients with cancer may be at a higher risk of contracting a COVID-19 infection, and if infected, may have poorer outcomes and an increased risk of mortality [1], [2]. As a consequence, there has been a rush to develop guidelines around every element of cancer care, including the use of surgery, radiation, systemic therapy, and supportive care modalities [3], [4], [5]. For survivors, cancer surveillance during the COVID-19 crisis has been the subject of intense debate. Through social media platforms, many physicians have touted therapeutic algorithms that attempt to account for the risk:benefit ratio of cancer treatment versus COVID-19 infection—although done with the best of intentions, the evidence base for these recommendations is admittedly weak.
In renal cell carcinoma (RCC), patients with localized disease are typically approached with resection followed by radiographic surveillance, with or without the application of adjuvant sunitinib [6], [7]. Patients on radiographic surveillance face potential for exposure to COVID-19 during their imaging procedures. Patients with advanced disease receive systemic treatment with targeted agents, immunotherapy, or a combination of both [8]. These patients face the dual risk of exposure during their treatment sessions and possible immunosuppression from their therapy. While physicians may offer recommendations for de-escalating therapy and surveillance in the face of COVID-19, decision making is ultimately a shared process with patients. To better understand shared decision making in the context of RCC, we conducted an online survey to ascertain (1) the patient anxiety level around COVID-19 and (2) its implications for preferred surveillance and treatment. This is a hypothesis-generating study, given the unprecedented nature of providing oncologic care in the midst of a pandemic.
2. Patients and methods
2.1. Survey development and distribution
The survey was developed by four members of the Kidney Cancer Research Alliance (KCCure) steering committee, with multidisciplinary representation from a surgeon (M.S.), a medical oncologist (S.K.P.), a psychologist (C.B.), and a patient advocate (D.B.). The survey included a total of 45 items (detailed subsequently) and was initially evaluated by a separate group of patient advocates for ease of interpretability. The survey was then broadcasted to the KCCure membership through a patient mailing list maintained by the organization and was also distributed through online social media platforms (specifically, Facebook, and Twitter).
2.2. Survey composition
The full survey is included in the Supplementary material. Briefly, the survey included demographic features such as age, gender, race, educational level, and income level. Patients were queried regarding their perceived risk of COVID-19, and their anxiety level related to both COVID-19 and cancer progression was quantified on a Likert scale ranging from 0 to 10. The receiver operating characteristic (ROC) curves identified the cutoff score of 4 for both scales (area under the curve = 0.74 and 0.75, respectively). Patients were asked whether they had communicated with their physician regarding the perceived risk related to COVID-19, and what information was conveyed. Multiple-choice questions were developed regarding perceived risk factors for COVID-19 infection, and patients were then queried regarding specific precautions they were taking.
Further questions were based on disease status. Patients in surveillance were queried regarding their current plan for surveillance, and to what extent (if at all) they were willing to delay scan assessment. Patients receiving systemic treatment were queried regarding the nature of systemic therapy that they were receiving and how often they currently saw their oncologist. Patients were then asked whether they would modify planned visits, scans, or infusions on account of COVID-19 risk, and whether changes in treatment algorithms were made on the basis of the pandemic.
The survey contained items pertaining to distress level and financial toxicity that will be detailed in future publications.
2.3. Statistical analysis
Descriptive statistics with graphical outputs were used to characterize survey results.
3. Results
3.1. Patient characteristics
Of 539 total respondents, 280 (52%) had metastatic disease, 187 (35%) had prior surgery for localized disease, and 23 (5%) had localized disease awaiting surgery (Table 1 ). The median age was 55 (range, 24–87) yr, with 58% being female and 39% males, and most patients were white (88%). Most patients had obtained a bachelor or graduate degree (44%) and lived in the USA (87%). In addition, the majority of patients were receiving treatment at an academic center (37%), followed by regional centers (30%) and private practices (18%).
Table 1.
Patient characteristics.
| Localized (no surgery; n = 23) | Localized (prior surgery; n = 187) | Metastatic (n = 280) | Total (n = 539) | |
|---|---|---|---|---|
| Age, median (range) | 46 (24–67) | 52 (26–86) | 57 (31–87) | 55 (24–87) |
| Gender, n (%) | ||||
| Male | 6 (26.1) | 49 (26.2) | 134 (47.8) | 189 (35.1) |
| Female | 14 (60.8) | 134 (71.6) | 137 (48.9) | 285 (52.8) |
| Race, n (%) | ||||
| White | 19 (82.6) | 165 (88.2) | 247 (88.2) | 473 (87.8) |
| Hispanic or Latino | 0 (0.0) | 7 (3.7) | 11 (3.9) | 20 (3.7) |
| Black/African American | 0 (0.0) | 4 (2.1) | 3 (1.1) | 7 (1.3) |
| Asian/Pacific Islander | 0 (0.0) | 6 (3.2) | 7 (2.5) | 17 (3.2) |
| Native American | 0 (0.0) | 2 (1.1) | 1 (0.3) | 3 (0.6) |
| Other | 0 (0.0) | 3 (1.6) | 5 (1.8) | 10 (1.9) |
| Type of practice, n (%) | ||||
| Academic center | 7 (30.4) | 57 (30.4) | 110 (39.3) | 199 (36.9) |
| Regional center | 7 (30.4) | 47 (25.1) | 98 (35.0) | 162 (30.1) |
| Community hospital | 3 (13.0) | 29 (15.5) | 30 (10.7) | 69 (12.8) |
| Private practice | 3 (13.0) | 53 (28.3) | 38 (13.6) | 98 (18.2) |
| Education level, n (%) | ||||
| Less than high school | 0 (0) | 3 (1.6) | 5 (1.8) | 9 (1.7) |
| High school | 3 (13.0) | 26 (13.9) | 41 (14.6) | 81 (15.0) |
| Some college | 6 (26.1) | 50 (26.7) | 65 (23.2) | 128 (23.7) |
| College/graduate degree | 10 (43.5) | 107 (57.2) | 164 (58.6) | 310 (57.5) |
| Income level ($), n (%) | ||||
| 0–24 999 | 3 (13.0) | 18 (9.6) | 20 (7.1) | 46 (8.5) |
| 25 000–49 999 | 4 (17.4) | 35 (1.6) | 39 (13.9) | 85 (15.8) |
| 50 000–99 999 | 6 (26.1) | 61 (32.6) | 85 (30.4) | 164 (30.4) |
| 100 000+ | 7 (30.4) | 60 (32.1) | 117 (41.8) | 203 (37.7) |
| Travel time to practice (h), n (%) | ||||
| 0–1 | 12 (52.2) | 121 (64.7) | 176 (62.8) | 338 (62.7) |
| 1–2 | 5 (21.7) | 40 (21.4) | 62 (22.1) | 116 (21.5) |
| 3–5 | 2 (8.7) | 14 (7.5) | 26 (9.3) | 47 (8.7) |
| 5+ | 1 (4.3) | 12 (6.4) | 12 (4.3) | 28 (5.2) |
3.2. COVID-19 concerns in patients with localized disease
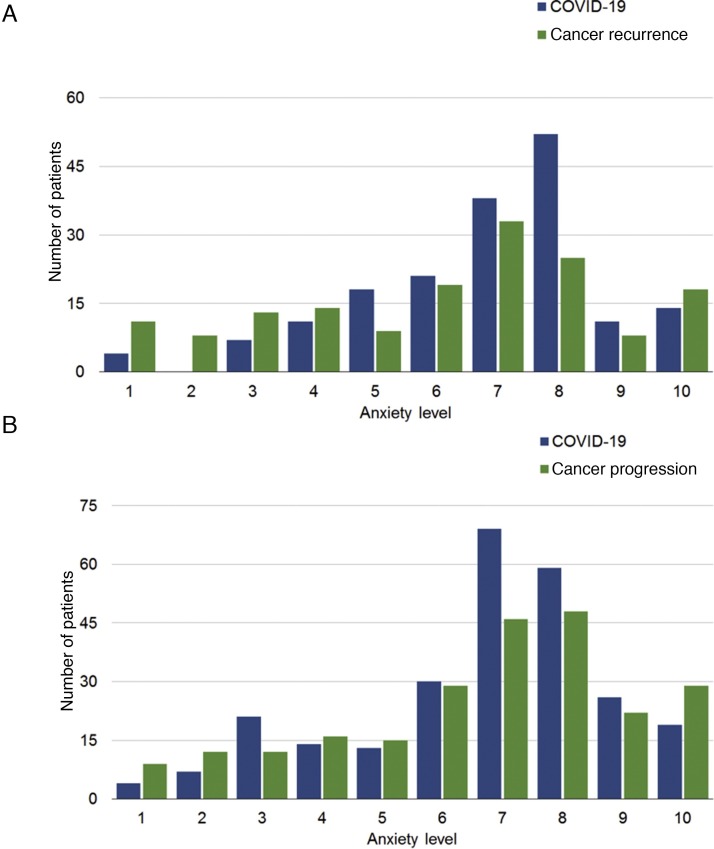
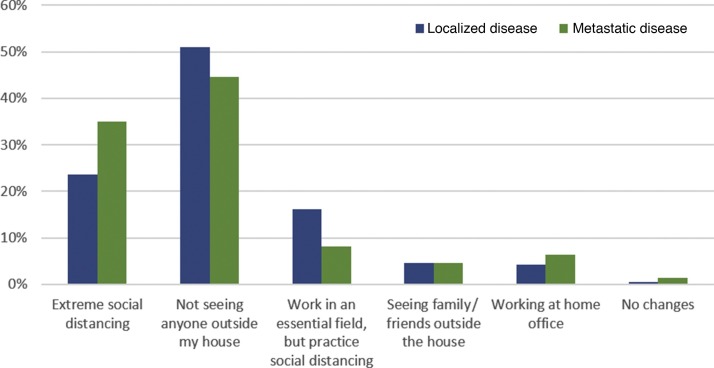
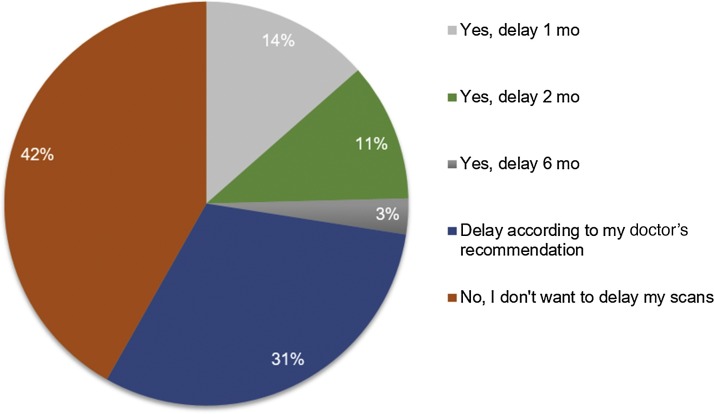
Among patients with localized disease who had received surgery, 170 (91%) were on surveillance, while 17 (9%) were on adjuvant therapy. Given the small sample of patients with localized disease who had not received surgery, our analysis focused on the much more sizeable population of patients who had received surgery, and were receiving either adjuvant therapy or surveillance. In this category, the median anxiety level related to COVID-19 infection was 7 (interquartile range, 6–8), while the median anxiety level related to cancer recurring was 7 (interquartile range, 4–8; Fig. 1 A). Utilizing this cutoff score, 94% of patients possessed moderate to severe symptoms of anxiety related to COVID-19, and 82% endorsed anxiety symptoms regarding their cancer diagnosis. Only a minority of patients (1%) reported no social distancing habits—a distribution of practices is seen in Figure 2 . Regarding barrier precautions, 33% and 45% reported wearing masks and gloves, respectively. Most patients were either very or somewhat willing to attend doctors’ appointments. Most patients (42%) had scans scheduled within the next 6 wk, with an additional 26%, 22%, and 10% having scans scheduled within 3 mo, 6 mo, and 1 yr, respectively. Most patients were unwilling to delay their visits for scan follow-ups (46%), although a minority of them were willing to delay by 1–2 mo (25%; Fig. 3 ).
Fig. 1.
Anxiety related to COVID-19 infection and cancer recurrence/progression in patients with localized disease following (A) surgery and (B) metastatic disease.
COVID-19 = coronavirus disease 2019.
Fig. 2.
Social distancing habits in patients with localized disease following surgery and metastatic disease.
Fig. 3.
Willingness of patients with localized disease following surgery to delay surveillance scans.
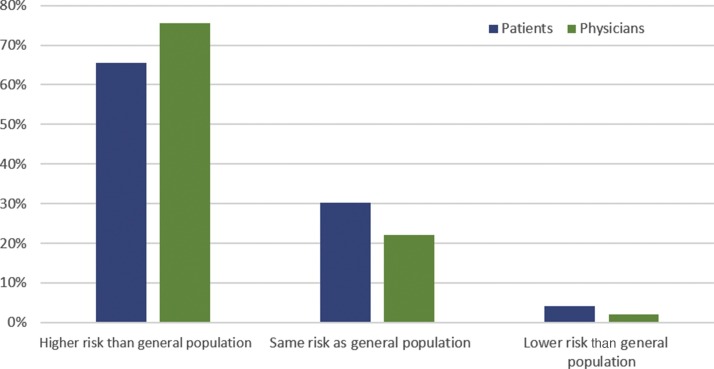
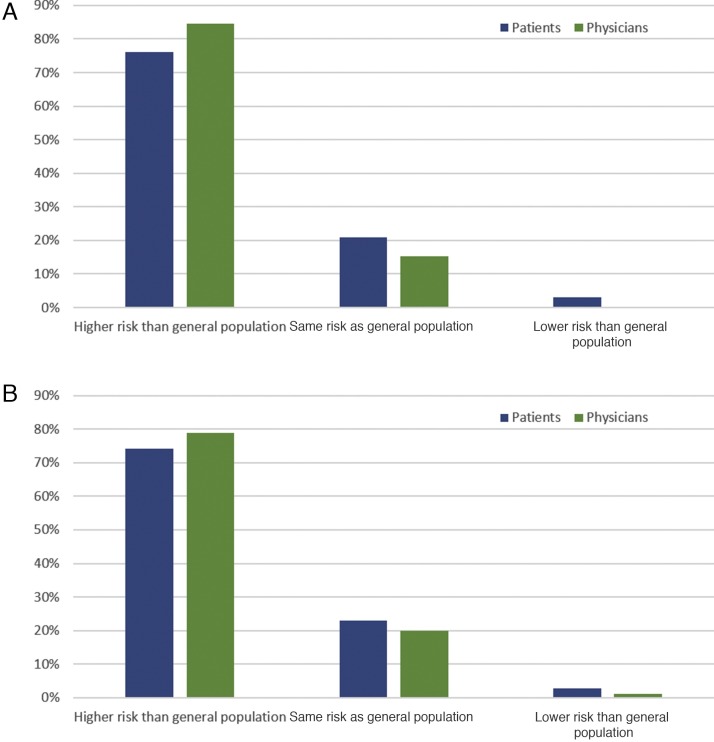
Among patients with localized disease with prior surgery, 26% had discussed their associated COVID-19 risk with their doctor; 76% were told by their physician that they had a higher risk of COVID-19 infection. A comparison of the physician- versus patient-perceived risk is shown in Figure 4 .
Fig. 4.
Patient- versus physician-perceived risk of COVID-19 in patients with localized disease following surgery.
COVID-19 = coronavirus disease 2019.
3.3. COVID-19 concerns in patients with metastatic disease
Among patients with metastatic disease, 215 (77%) were on systemic therapy, while 65 (23%) had not yet received systemic treatment. In patients with metastatic disease receiving therapy, the most common treatments were nivolumab monotherapy (22%), cabozantinib (17%), pembrolizumab in combination with axitinib (13%), and nivolumab in combination with ipilimumab (12%). In total, 91 patients (42%) were receiving targeted therapy, 78 (36%) were receiving immunotherapy, and 46 (22%) were receiving a combination. In this category, the median anxiety level related to COVID-19 infection was 7 (interquartile range, 6–8), while the median anxiety level related to cancer progression was 7 (interquartile range, 5–8; Fig. 1B), which means that 88% possessed symptoms of anxiety related to COVID-19 and 88% had symptoms associated with their cancer diagnosis. As with patients with localized disease, only a small minority of patients with metastatic disease did not practice social distancing (1%; Fig. 2). A larger proportion of patients with metastatic disease (vs those with localized disease) used masks and gloves (46% and 49% vs 33% and 45%, respectively).
Patients with metastatic disease who were not on systemic therapy most commonly saw their physician every 3–6 mo (54%), while patients on active systemic therapy frequently saw their physician at intervals of ≤1 mo (77%). Most of the patients (64%) on systemic therapy were unwilling to pause their therapy. Among patients receiving infusion therapy, 85% reported that they would either be very unwilling or be unwilling to skip an infusion. Few patients reported treatment delays/pauses by their oncologist—of 14 patients who reported treatment delays, five reported a delay in targeted therapy administration, nine reported a delay in immunotherapy administration, and two reported a delay in both.
Among patients with metastatic disease, 114 (40%) had discussed with their doctor about their associated COVID-19 risk. Of the 91 patients receiving targeted therapy, those who reported a conversation with their physician were more often told that they had a higher risk of COVID-19 infection (72%) versus a neutral (18%) or a decreased (0%) risk. Of the 124 patients receiving immunotherapy, those who reported a conversation with their physician were also more often told that they had a higher risk of COVID-19 infection (65%) versus a neutral (18%) or a decreased (2%) risk (Fig. 5 ).
Fig. 5.
Patient versus physician-perceived risk of COVID-19 infection in (A) patients with metastatic disease and (B) patients with metastatic disease receiving systemic therapy.
COVID-19 = coronavirus disease 2019.
4. Discussion
Our study reveals that while patients experience heightened anxiety related to COVID-19, they remain substantially concerned about cancer recurrence and progression. The proportion of patients with moderate to severe anxiety related to COVID-19 or their cancer diagnosis was notably higher than that reported in previous studies [9]. As such, we observed that many patients with RCC are unwilling to compromise planned surveillance for localized disease or planned systemic therapy for metastatic disease. In the overall population, 71% felt that they had a heightened risk for COVID-19 infection; however, only 27% of patients contacted their treating physicians to confirm this information. A large proportion of patients with both localized and metastatic disease exhibited some social distancing measures, with 30% self-quarantining to mitigate the COVID-19 risk.
The data also highlight varied responses from providers and patients regarding the impact of COVID-19 on systemic therapy. A cohort study that evaluated the clinical impact of COVID-19 on patients with cancer has noted that race/ethnicity, obesity, cancer type, and type of therapy were not associated with mortality [2]. However, older age, performance status, patients on therapy, and the presence of any comorbidities were associated with an increased risk of death [2], [10]. Furthermore, a detailed review compiled by the European Society of Clinical Microbiology and Infectious Diseases suggested a higher risk of infection with vascular endothelial growth factor (VEGF)-directed therapies, likely linked to a higher rate of neutropenia [11]. However, there are data to implicate that many VEGF tyrosine kinase inhibitors (namely axitinib, cabozantinib, lenvatinib, sunitinib, and pazopanib) stimulate natural killer cells and induce CD8+ T cells, thus contributing to increased immune reactivity [12]. Similar to targeted therapy, the impact of checkpoint inhibitors on infectious disease is equally controversial. Studies on hepatitis B virus infection have identified that PD-1+ CD8 T cells have impaired function and are marked for apoptosis [13]. In hepatitis C virus infection, liver biopsy show high levels of both PD-1+ CD4 and PD-1+ CD8 T cells [14]. Although these findings might imply a role of PD-1 blockade in viral infection, there is only anecdotal evidence of this [15], [16]. In fact, there are several reports suggesting that patients receiving checkpoint blockade for cancer may be at a heightened risk for both bacterial and viral infections, as opposed to having a protective effect [17]. With these conflicting data in mind, it is no surprise that both patients and providers have conflicting views regarding the risk of COVID-19 in association with systemic therapy.
Limitations of the study include the use of data supplied by patients. Using this approach, confirmation of medical data (eg, histology, stage, and treatment regimen) is not feasible. In addition, certain elements are challenging to clarify—for instance, in physician-assessed risk of COVID-19 infection, it is unclear whether a medical oncologist, urologist, or primary care physician served as a source. There is also likely some selection bias among survey respondents—our population was highly educated and primarily based in the USA, and a relatively high proportion were treated at academic centers. Although we used a nonvalidated survey in the current study, the ROC curve was able to better identify symptoms of anxiety among these patients with RCC. Furthermore, this is a hypothesis-generating study. Perhaps most importantly, the data for COVID-19 are evolving extremely rapidly. The distribution of cases is changing, as is the approach to infection prevention, prophylaxis, and treatment [18], [19], [20]. As such, it is possible that the perspective of patients and physicians early in the pandemic could evolve drastically as the situation progresses. Important efforts are underway to specifically characterize the impact of COVID-19 in patients with cancer—the American Society of Clinical Oncology has launched a survey of providers to ascertain the patterns of care in infected patients with cancer. Registries such as this and prospective clinical trials may inform best practices. To supplement this, we plan to continue to survey the RCC community to obtain the patient perspective on management.
5. Conclusions
Our data highlight an anxiety pertaining to COVID-19 that is counterbalanced by significant concern around RCC recurrence and progression. Most patients are reluctant to alter previously instituted plans for RCC surveillance or treatment. As governments and medical societies rapidly construe clinical guidelines in the era of COVID-19, it is critical to assess the patient voice. While hospitals are making decisions based on scarce resources and societal needs, the theme of individualized patient care should not be sacrificed.
Author contributions: Cristiane Decat Bergerot had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Staehler, Battle, Pal, Bergerot.
Acquisition of data: Staehler, Battle, Pal, Bergerot.
Analysis and interpretation of data: Staehler, Battle, Pal, Bergerot.
Drafting of the manuscript: Staehler, Battle, Pal, Bergerot.
Critical revision of the manuscript for important intellectual content: Staehler, Battle, Pal, Bergerot.
Statistical analysis: Staehler, Pal, Bergerot.
Obtaining funding: None.
Administrative, technical, or material support: Staehler, Battle, Pal, Bergerot.
Supervision: Staehler, Pal, Bergerot.
Other: None.
Financial disclosures: Cristiane Decat Bergerot certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Cristiane Decat Bergerot and Dena Battle report no conflicts of interest. Professor Dr. Michael Staehler is a company consultant for Pfizer, Novartis, GlaxoSmithKline, Roche, Astellas, and Bayer; has received company speaker honoraria from Pfizer, Novartis, GlaxoSmithKline, Roche, Astellas, Bayer, and Aveo; has participated in trials for Pfizer, Novartis, GlaxoSmithKline, Roche, Bayer, Aveo, Wilex, and Immatics; has received fellowships and travel grants from Pfizer, Novartis, GlaxoSmithKline, Roche, and Bayer; has received grants/research support from Pfizer, Novartis, GlaxoSmithKline, Roche, Bayer, and Aveo; and, in addition, took part in the S-TRAC trial as an investigator and is an author of the S-TRAC publication. Sumanta Kumar Pal reports personal fees from Pfizer, Novartis, AVEO, Myriad Genetics, Genentech, Exelixis, BMS, Astellas, and from Medivation.
Funding/Support and role of the sponsor: None.
Acknowledgments: The authors would like to thank all patients and their caregivers for their significant contributions in sharing their own experience through the cancer journey.
Associate Editor: Christian Gratzke
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.euf.2020.09.002.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Liang W., Guan W., Chen R., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ueda M., Martins R., Hendrie P.C., et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward a common goal. J Natl Compr Canc Netw. 2020;18:1–4. doi: 10.6004/jnccn.2020.7560. [DOI] [PubMed] [Google Scholar]
- 4.Yang G., Zhang H., Yang Y. Challenges and countermeasures of integrative cancer therapy in the epidemic of COVID-19. Integr Cancer Ther. 2020;19 doi: 10.1177/1534735420912811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagano M.B., Hess J.R., Tsang H.C., et al. Prepare to adapt: blood supply and transfusion support during the first 2 weeks of the 2019 novel coronavirus (COVID-19) pandemic affecting Washington State. Transfusion. 2020;60:908–911. doi: 10.1111/trf.15789. [DOI] [PubMed] [Google Scholar]
- 6.Dabestani S., Beisland C., Stewart G.D., et al. Long-term outcomes of follow-up for initially localised clear cell renal cell carcinoma: RECUR database analysis. Eur Urol Focus. 2019;5:857–866. doi: 10.1016/j.euf.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Ljungberg B., Albiges L., Abu-Ghanem Y., et al. European Association of Urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol. 2019;75:799–810. doi: 10.1016/j.eururo.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Choueiri T.K., Motzer R.J. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. 2017;376:354–366. doi: 10.1056/NEJMra1601333. [DOI] [PubMed] [Google Scholar]
- 9.Bergerot C.D., Battle D., Staehler M.D., Pal S.K. Distress in patients with renal cell carcinoma: a curious gap in knowledge. BJU Int. 2019;123:208–209. doi: 10.1111/bju.14564. [DOI] [PubMed] [Google Scholar]
- 10.Garassino M.C., Whisenant J.G., Huang L.C., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancer Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguilar-Company J., Fernández-Ruiz M., García-Campelo R., Garrido-Castro A.C., Ruiz-Camps I. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (cell surface receptors and associated signaling pathways) Clin Microbiol Infect. 2018;24:S41–52. doi: 10.1016/j.cmi.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 12.Santoni M., Berardi R., Amantini C., et al. Role of natural and adaptive immunity in renal cell carcinoma response to VEGFR-TKIs and mTOR inhibitor. Int J Cancer. 2014;134:2772–2777. doi: 10.1002/ijc.28503. [DOI] [PubMed] [Google Scholar]
- 13.Ye B., Liu X., Li X., Kong H., Tian L., Chen Y. T-cell exhaustion in chronic hepatitis B infection: current knowledge and clinical significance. Cell Death Dis. 2015;6:e1694. doi: 10.1038/cddis.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasprowicz V., zur Wiesch J.S., Kuntzen T., et al. High level of PD-1 expression on hepatitis C virus (HCV)-specific CD8+ and CD4+ T cells during acute HCV infection, irrespective of clinical outcome. J Virol. 2008;82:3154–3160. doi: 10.1128/JVI.02474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyck L., Mills K.H.G. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur J Immunol. 2017;47:765–779. doi: 10.1002/eji.201646875. [DOI] [PubMed] [Google Scholar]
- 16.Koralnik I.J. Can immune checkpoint inhibitors keep JC virus in check? N Engl J Med. 2019;380:1667–1668. doi: 10.1056/NEJMe1904140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujita K., Kim Y.H., Kanai O., Yoshida H., Mio T., Hirai T. Emerging concerns of infectious diseases in lung cancer patients receiving immune checkpoint inhibitor therapy. Respir Med. 2019;146:66–70. doi: 10.1016/j.rmed.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Fauci A.S., Lane H.C., Redfield R.R. COVID-19—navigating the uncharted. N Engl J Med. 2020;382:1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao B., Wang Y., Wen D., et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parmet W.E., Sinha M.S. Covid-19—the law and limits of quarantine. N Engl J Med. 2020;382:e28. doi: 10.1056/NEJMp2004211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.