Fig. 4. MAP4K4/6/7 kinases phosphorylate ubiquitin in vitro and in cells.
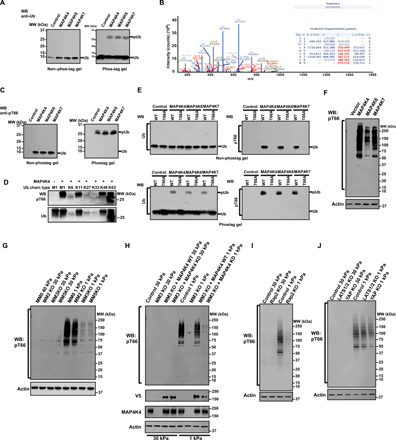
(A) An in vitro kinase assay was performed at 30°C for 1 hour in the presence of free Ub and indicated purified kinases. Samples were run on non–phos-tag or phos-tag PAGE and the gels were blotted with anti-Ub antibody. WB, Western blotting; MW, molecular weight. (B) Gel band of phosphorylated ubiquitin was trypsinized and subjected to LC-MS/MS analysis. (C) Samples were processed as in (A) and blotted with anti-pT66 antibody. (D) An in vitro kinase assay was performed with different tetra-Ub chains and MAP4K4. The gels were subjected to Western blot with anti-pT66 antibody (top) or anti-Ub antibody (bottom). (E) An in vitro kinase assay was performed with indicated Ub proteins and purified kinases. Samples were run on non–phos-tag or phos-tag PAGE, and the gels were blotted with anti-Ub or anti-pT66 antibody. WT, wild type. (F) HEK293 cells grown on plastic plates were transfected with the indicated constructs, and phosphorylation of ubiquitin in cells was detected with anti-pT66 antibody. Actin was used as a loading control. (G to J) Indicated cells were cultured on low (1 kPa) and high (30 kPa) stiffness fibronectin-coated hydrogels for 24 hours. Cell lysates were probed with indicated antibodies. Actin was used as loading control.
