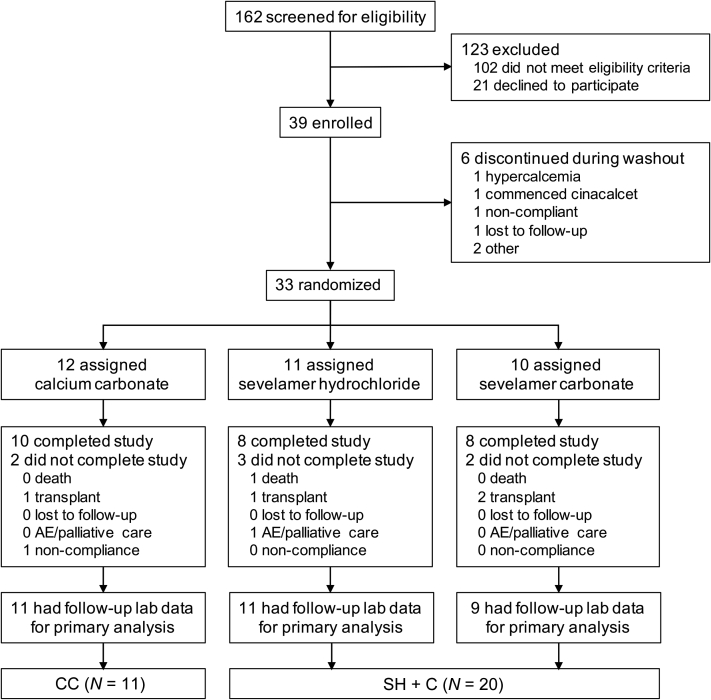
Figure 1.
CONSORT diagram showing patient disposition through consent, randomization, and dropout in the Sevelamer Versus Calcium to Reduce Fetuin-A-containing Calciprotein Particles in Dialysis (SCaRF) trial. Primary analyses were conducted on a modified intention-to-treat basis in which randomized patients with at least 1 postrandomization follow-up visit were included. AE, adverse events; CC, calcium carbonate; SC, sevelamer carbonate; SH, sevelamer hydrochloride.