Figure 6.
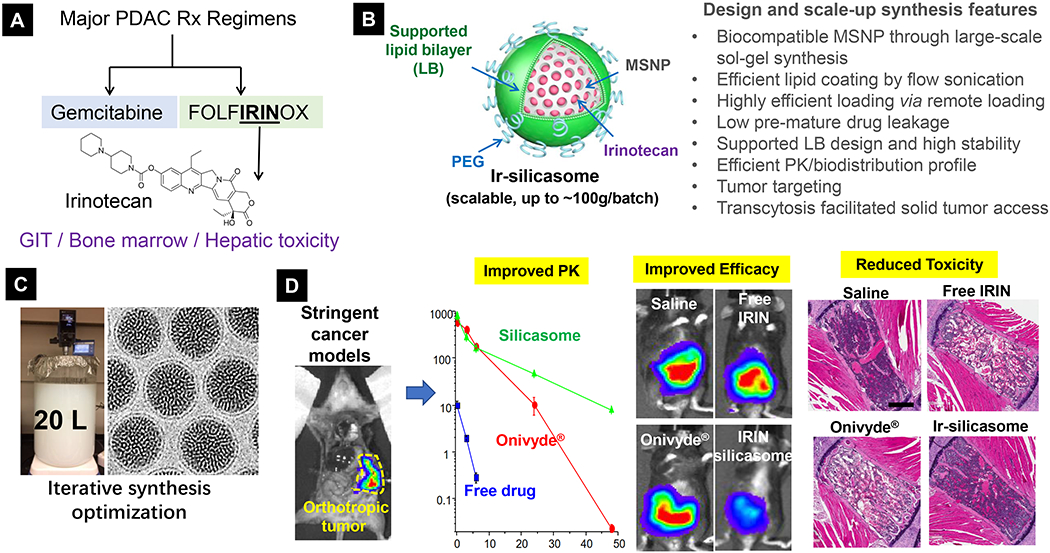
Development of silicasome drug nanocarrier to address irinotecan safety and efficacy for pancreatic and colon cancer treatment. (A) In the setting of PDAC, while FOLFIRINOX is more potent than GEM, this regimen is far more toxic, in which irinotecan contributes in a major way to its toxicity on the bone marrow and the GIT. (B) Scheme and unique characteristics of silicasome nanocarrier. (C) Successful scale-up development of silicasome for translational study. (D) Use of highly stringent orthotopic cancer models to study PK, efficacy and safety features of silicasome nanocarrier. Irinotecan delivery by silicasome led to a major improvement of efficacy and safety over free drug and commercial liposomes for PDAC and colon cancer. In order to study irinotecan’s toxicity in diffident formulations, liver, sternal bone marrow, and intestinal tissues were collected from the mice. Histological examination of these organs showed reduced hepatocyte necrosis and GIT apoptosis. Panel D highlights the H&E staining result of bone marrow. While animals treated with free drug or commercial liposomes generated strong effects on bone marrow damage (i.e. evidenced by ~30% of the space being filled by hematopoietic cells), there was no major reduction in silicasome treated mice. This correlates to peripheral blood neutropenia in patients receiving either free drug or Onivyde®. No neutropenia was observed in mice receiving irinotecan silicasome. Reproduced with permission.[155, 164] Copyright 2017, American Society for Clinical Investigation. All other figures. Copyright 2019, American Chemical Society.
