Extended Data Figure 6: Representative map quality and model fit and structural analysis of GABA alone, diazepam and flumazenil complexes.
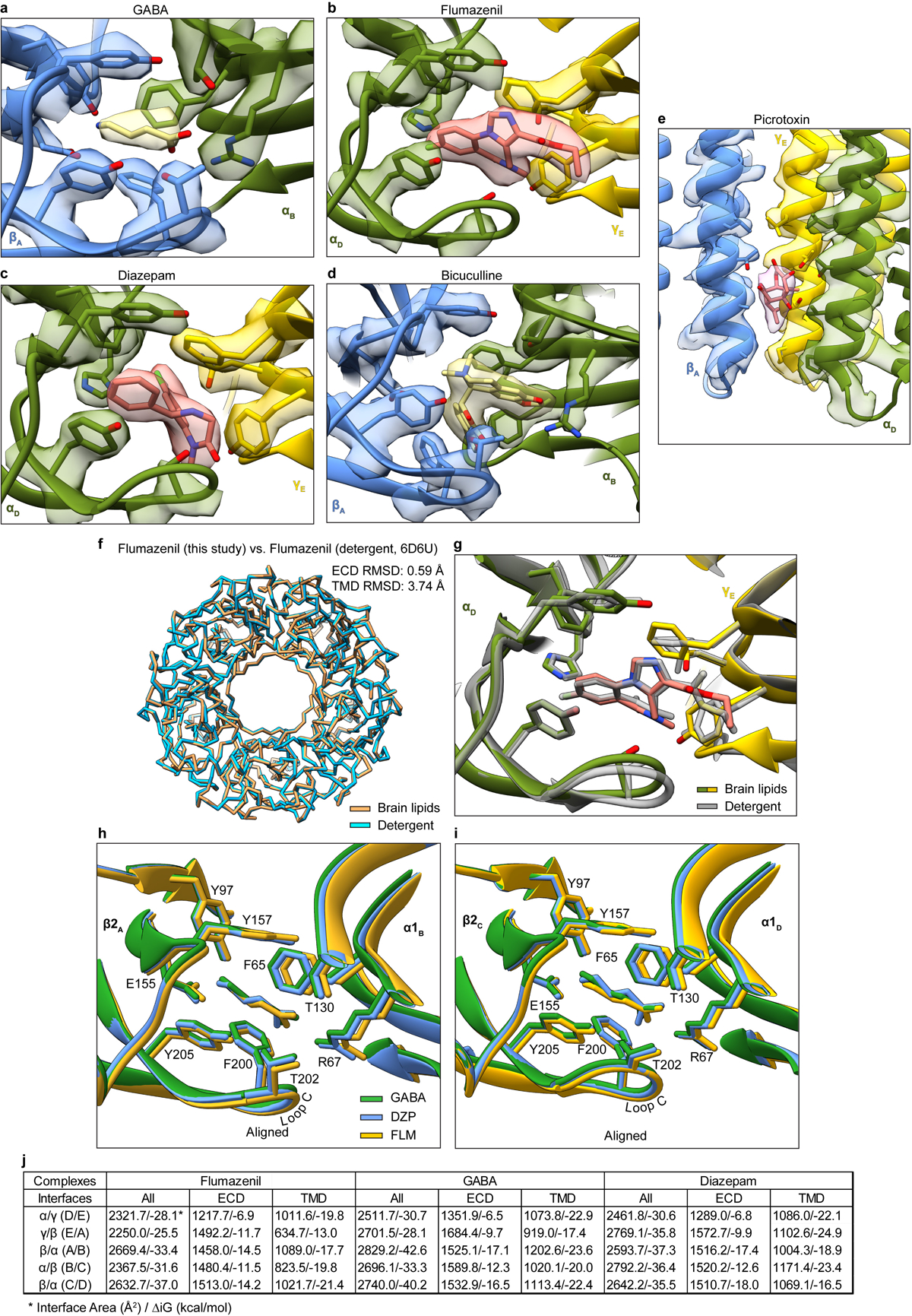
Semitransparent surface is shown for central ligand and contacting side chains for panels a-d. Panel a shows GABA site at chain A-B β-α interface in GABA alone structure. The two β-α GABA sites from the structure superimpose nearly perfectly and do not shed light on the differences in functional contributions found in electrophysiology studies with concatamers48. Structures of apo receptor may be essential in identifying structural differences in the two GABA sites. Panel b shows flumazenil site at α-γ interface; panel c shows diazepam at same ECD interface in its structure. Panel d shows bicuculline site at same interface as panel a. Panel e shows picrotoxin site in TMD; here, density is shown for ligand and all nearby protein structure elements. Panel f shows superposition of two GABA plus flumazenil complexes, one from the detergent condition21 and one from this study in brain lipids, to illustrate absence of differences in backbone conformation. Note, loops that interact with the TMD do vary in conformation. Panel g shows detail of flumazenil site from the superposition in panel f. Panels h and i show superpositions of three structures from the current study: GABA alone, GABA plus diazepam and GABA plus flumazenil, focused on the two GABA binding sites. Panel j shows calculated interface areas and interaction energies for each subunit pair, for each of the benzodiazepine-related structures.
