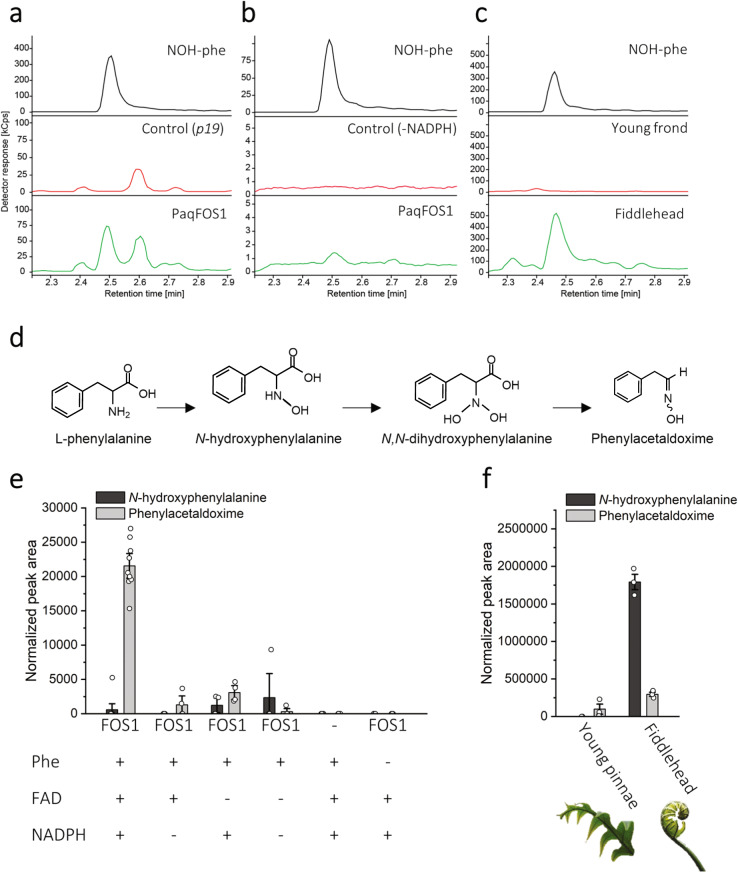
Fig. 6. The formation of N-hydroxyphenylalanine (NOH-phe).
LC–MS chromatograms from targeted analysis of samples compared with the authentic standard, derived from a transient expression of FOS1 in N. benthamiana showing the p19 as a negative control, b heterologous expression of FOS1 in E. coli using the absence of NADPH as a negative control, and c the presence of N-hydroxyphenylalanine in P. aureum tissue. The chromatographic trace represents the abundance of the fragment ion of N-hydroxyphenylalanine (precursor ion → fragment ion of 182.1 → 136.0; see Supplementary Table 4). d The hypothesized biosynthetic route from phenylalanine to phenylacetaldoxime, as catalyzed by FOS1. e In vitro activity assay of recombinant PaqFOS1 using l-phenylalanine (Phe) as a substrate, with different combinations of the necessary cofactors FAD and NADPH. Bars represent mean ± SE (n ≥ 3). f The presence of N-hydroxyphenylalanine in P. aureum fiddlehead and young pinnae metabolite extracts (n = 3).