Abstract
Purpose
To describe a case of plateau iris syndrome (PIS) and angle-closure glaucoma (ACG) in a patient with nail-patella syndrome (NPS).
Observation
A 33 year-old woman of Slovakian ancestry from Norway with a history of NPS presented with angle-closure secondary to plateau iris. At the time of her NPS diagnosis, she had no ocular pathology. Genetic testing revealed a rare de novo mutation in LMX1B [c.668G>C (p.Arg223Pro)]. Two years later, she experienced acute bilateral ocular pain and blurred vision in the setting of one year of reported visual loss. Subsequent ophthalmic examinations revealed closed angles and plateau iris OU with ACG OD and angle-closure OS. Perimetry showed superonasal visual field defects OD and no defects OS. Ocular coherence tomography (OCT) revealed thinning of the inferior pole of the optic nerve OD. Medical management proved ineffective. A laser peripheral iridotomy (LPI) OD was performed, without resolution of the angle-closure, and a diagnosis of plateau iris syndrome (PIS) was made. She was then treated with an argon laser peripheral iridoplasty (ALPI) and clear lens extraction with a posterior chamber intraocular lens (PCIOL) and goniosynechialysis OD, but her IOP remained elevated OU. She was referred to New York Eye and Ear Infirmary of Mount Sinai, where an LPI OS was performed, but angle-closure persisted, consistent with PIS. An ALPI OS with touch-up was performed, and her IOP normalized with dark-room gonioscopy revealing open angles OU.
Conclusions And importance
NPS has been associated with ocular hypertension (OHTN) and open-angle glaucoma (OAG); however, to our knowledge, no association between NPS and angle-closure has previously been reported. The case described here, of a patient with a rare de novo mutation and ocular findings of PIS with associated ACG, represents a novel genetic and clinical presentation of NPS.
Keywords: Nail-patella syndrome, Plateau iris, Angle-closure glaucoma, LMX1B
1. Introduction
Nail-patella syndrome (NPS) is a rare pleiotropic autosomal dominant condition, with a reported incidence of 1 in 50,000 live births.1 It primarily affects the integumentary and musculoskeletal systems; however, other body systems, including the eye, and renal and neurologic systems, are frequently involved.1,2 The classic clinical tetrad of NPS, some of which was first described in the late 1800s, consists of abnormalities of the nails, knees, elbows, and iliac bones.1, 2, 3, 4 In 1998, the LIM-homeodomain gene LMX1B was identified as the gene responsible for NPS.5,6 The encoded transcription factor, LMX1B, plays a role in dorsoventral patterning in the developing limb,7, 8, 9 differentiation of the anterior segment of the eye,10 glomerulogenesis,11, 12, 13, 14 and the development of certain neuronal populations.15, 16, 17 Haploinsufficiency of this transcription factor has been implicated in the pathogenesis of NPS.5,6,18, 19, 20
NPS is a highly penetrant disorder with variable expressivity, presenting with varied intra- and interfamilial frequency and severity.1 Common manifestations include hypoplastic nails, triangular or absent nail lunulae, patellar hypoplasia, reduced elbow range of motion, iliac horns, lean body habitus, difficulty gaining weight, low muscle mass in the proximal extremities, poor breast development, loss of distal interphalangeal (DIP) skin creases, “swan necking” of the fingers, talipes equinovarus, back pain, and renal, gastrointestinal, neurologic, vasomotor, and dental defects.1 Radiologic findings include hypoplasia of the lateral humeral epicondyles and capitellum, prominent medial humeral epicondyles, and dysplasia and dislocation of the radial head.1
Ocular abnormalities have also been documented in patients with NPS.14,20, 21, 22, 23 Ocular hypertension (OHTN) and open-angle glaucoma (OAG) are the primary manifestations, with a reported prevalence of approximately 7.2% and 9.6%, respectively.1 In patients over age 40, these values increased to 11.9% and 16.7%, respectively.1 In a study of 24 patients from two families, Lichter et al described cosegregation of NPS and OAG in 50% of NPS patients, providing evidence for a genetic basis for their association.21 Other reported ocular abnormalities include microcornea, sclerocornea, congenital cataracts, iris processes, and cloverleaf pigmentation of the inner margin of the iris (“Lester's sign”).4 However, to our knowledge, an association between NPS and plateau iris syndrome (PIS) and/or angle-closure glaucoma (ACG) has not been previously reported. We describe a patient with NPS who has ACG associated with PIS.
2. Case presentation
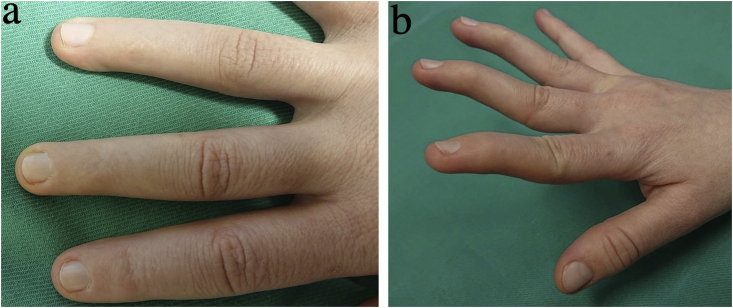
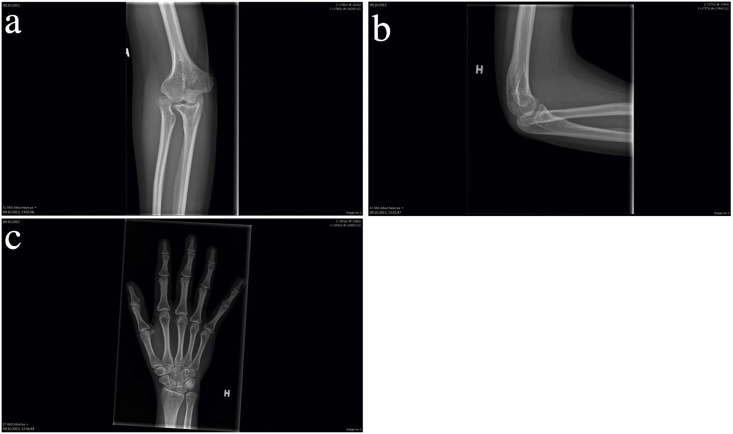
In April 2017, a 33 year-old woman of Slovakian ancestry with a history of NPS was referred from Norway to the New York Eye and Ear Infirmary of Mount Sinai for treatment of angle-closure secondary to PIS. She was officially diagnosed with NPS in 2014, presenting with pathognomonic features including hypoplastic fingernails with absent lunulae on fingers 2–5 in both hands (Fig. 1), dysplastic toenails on toes 2–5, and patellar hypoplasia. Other clinical manifestations included swan-neck finger deformities and hypomobile DIP joints with loss of overlying skin creases in fingers 2–4 on both hands (Fig. 1), low upper body muscle mass, poor breast development, reduced appetite, difficulty gaining weight, limited elbow extension, pronation, and supination, surgically corrected talipes equinovarus, and generalized musculoskeletal pain. Radiologic findings included hypoplasia of the lateral humeral epicondyles and dislocation of the radial heads, dysplasia of the distal ulnae with absent ulna styloid processes and minimal ulna plus variance (Fig. 2), shortened first metacarpal and second and fifth intermediate phalanges, and reduced height of the tarsometatarsal joints spaces with small bony accumulations in the first tarsometatarsal joint. Other than low hyperopia requiring glasses at age 10, she had no prior history of ocular disease. Her other medical history included depression, treated with mirtazapine.
Fig. 1.
Clinical features of nail-patella syndrome. (a) Hypoplastic nails with absent lunulae and loss of distal interphalangeal skin creases on fingers 2–4. (b) Swan-neck deformities of fingers 2–4.
Fig. 2.
Radiologic features of nail-patella syndrome, right upper extremity. (a) Hypoplasia of the lateral humeral epicondyle, dislocation of the radial head, and dysplasia of the distal ulna with an absent ulna styloid process. (b) Shortened first metacarpal and second and fifth intermediate phalanges.
Her family history was negative for NPS or other musculoskeletal or ocular diseases. Genetic testing in 2014 revealed a rare de novo mutation [c.668G>C (p.Arg223Pro)] in the LMX1B gene. This mutation was not observed in any of the >100,000 participants from the gnomAD database, which aggregates whole exome and whole genome sequencing data from participants of African, Asian, European, and Latino ancestry.24 Subsequent renal testing revealed no abnormalities.
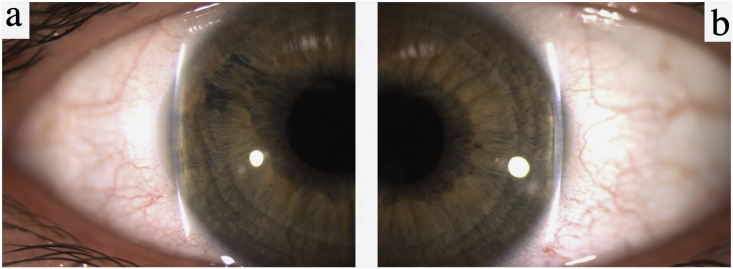
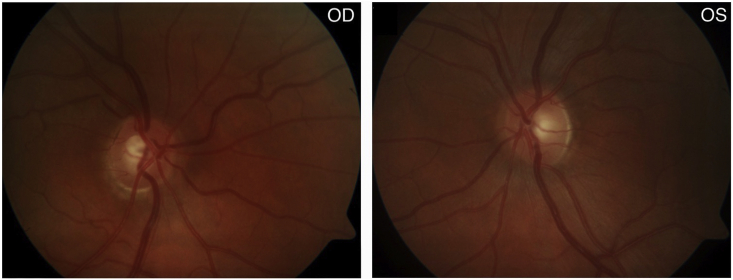
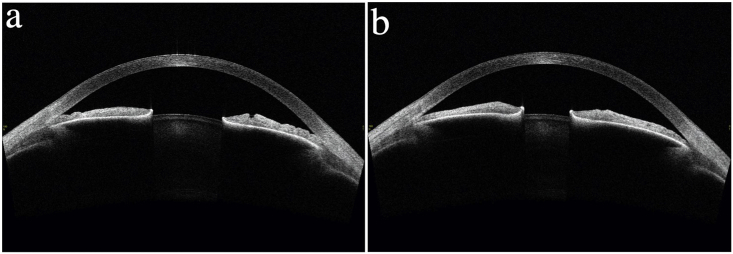
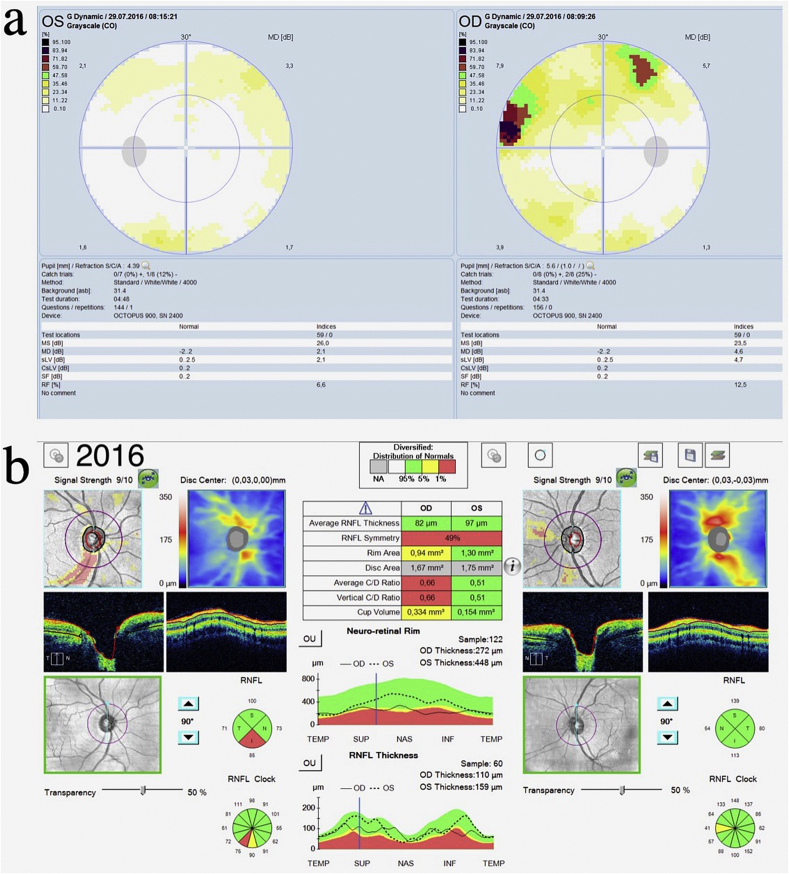
In June 2016, she presented in Slovakia with acute eye pain, blurred vision, and elevated intraocular pressures (IOPs) of 54 mmHg OD and 37 mmHg OS due to acute angle-closure OU, in the setting of one year of reported visual loss. She was diagnosed with ACG OD and angle-closure OS and started on dorzolamide-timolol OU. A week later at a follow-up visit in Norway, her IOPs had decreased to 21 mmHg OD and 16 mmHg OS. Her best corrected visual acuity (BCVA) at this time was 0.9 (+2.25–1.0 x 99) OD and 1.0 (+1.25–0.5 x 80) OS. Her anterior chamber depth and axial length measurements were 2.7 mm OU and 21.9 mm OU, respectively. Corneal thickness was 540 μm OU. Her slit lamp exam revealed narrow angles OU via the Van Herick technique (Fig. 3), and an increased cup-to-disc ratio OD (Fig. 4). Gonioscopy and anterior segment ocular coherence tomography (OCT) also revealed very narrow angles OU, which opened more with pilocarpine (Fig. 5). Dynamic gonioscopy revealed possible plateau iris configuration (PIC) OU and extensive peripheral anterior synechiae (PAS) temporally OD. Her visual field test and retinal OCT showed superonasal glaucomatous visual defects OD and thinning of the inferior pole of the optic nerve OD, respectively, with normal perimetry and OCT OS (Fig. 6).
Fig. 3.
Slit lamp exam: Van Herick technique (June 2016). (a) Van Herick grading revealing a narrow angle and superotemporal anterior synechia OD. (b) Van Herick grading revealing a narrow angle OS.
Fig. 4.
Disc photos (June 2016). Inferior cupping of the optic nerve head OD.
Fig. 5.
Anterior segment ocular coherence tomography (Tomey AS-OCT - Japan) image OS (June 2016). (a) Narrow angles. (b) Pupillary constriction with partial opening of the iridocorneal angle temporally after administration of topical pilocarpine.
Fig. 6.
Octopus visual fields and ocular coherence tomography (June 2016). (a) Perimetry reveals superonasal glaucomatous visual defects OD, with normal perimetry OS. (b) Ocular coherence tomography reveals thinning of the inferior pole of the optic nerve OD.
After failed trials of brinzolamide OU and pilocarpine 2% OS, she was treated with a YAG laser peripheral iridotomy (LPI) OD (8/2016), without anatomical or functional effect. She continued to experience pain with accommodation, and with dim light testing had IOP spikes of >50 mmHg OU, consistent with a diagnosis of PIS. A trial of reading glasses failed to control these IOP spikes. A repeat LPI OD was performed, followed by a 180° argon laser peripheral iridoplasty (ALPI) OD using settings of 500-μm spot size, 0.5-s-duration, and 240-mW power. She continued to experience IOP elevations, and underwent clear lens extraction with a posterior chamber intraocular lens (PCIOL) and goniosynechialysis OD (10/2016), complicated by persistent low-grade chronic uveitis. She was started on brinzolamide-timolol and chloramphenicol-dexamethasone daily OD and pilocarpine 0.5% TID OS, but her IOPs continued to fluctuate, with elevations up to 45 mmHg OU. Her treatment regimen was adjusted to pilocarpine 2% QID OU and chloramphenicol-dexamethasone BID OD.
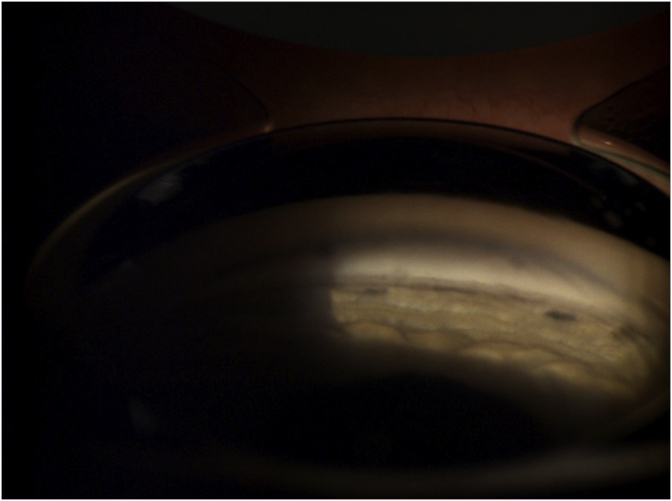
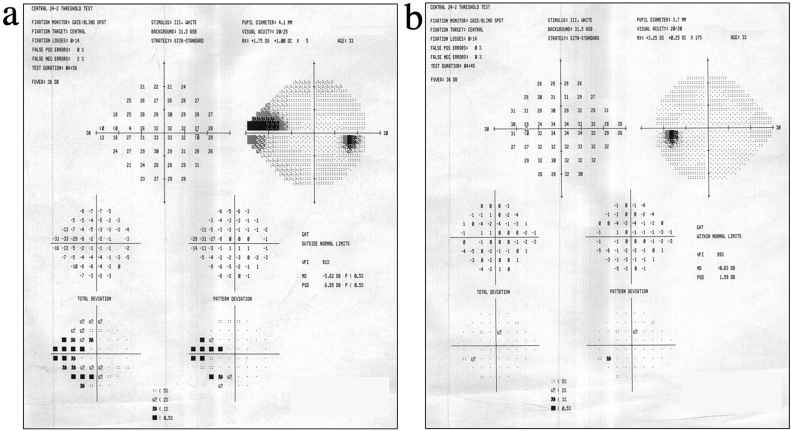
At this point, she was referred to the New York Eye and Ear Infirmary of Mount Sinai (NYEE) for further treatment. Due to difficulties effectively treating the right eye, treatment of the left eye was delayed until the patient was able to come to New York for treatment. Upon presentation at NYEE, her BCVA was 20/25 (−1.25 + 1.00 x 175) OD and 20/20 (+0.25 + 0.25 x 175) OS. Her IOPs were 18 mmHg OD and 16 mmHg OS, prior to using her morning drops. Her slit lamp exam showed evidence of a patent LPI and a PCIOL OD, with evidence of inferior ONH cupping OD. Dark-room gonioscopy OD revealed a Shaffer grade III angle inferiorly and nasally with PAS to the lower pigmented trabecular meshwork (TM), slit superiorly, and anterior iris insertion, mostly to the base of the PTM, with iris adhesions to the cornea superotemporally. Dark-room indentation gonioscopy OS revealed the angle to be closed to the upper trabecular meshwork for 360°, opening to the scleral spur with indentation, with increased pigment and PAS to the mid-pigmented meshwork superiorly. A prominent double hump sign and a Fuji sign (indicative of a lens-related component) were present OS (Fig. 7). 24-2 SITA Standard Humphrey Visual Field testing showed superonasal and inferonasal defects OD and was normal OS (Fig. 8).
Fig. 7.
Gonioscopic view OS after indentation, photographed status post LPI and ALPI. Reveals characteristic double hump sign due to plateau iris.
Fig. 8.
24-2 SITA Standard Humphrey Visual Fields (April 2017). Reveals superonasal and inferonasal defects OD (a) with normal perimetry OS (b).
A LPI OS was performed, with a post-treatment IOP of 12 mmHg OU. She was started on pilocarpine 2% TID OD, and prednisolone QID OS. Two days later, her IOP measurements were 30 mmHg OD and 34 mmHg OS. She reported non-adherence to pilocarpine due to adverse effects, including headaches, ocular itchiness, and blurred vision. Gonioscopy OD showed no change, and gonioscopy OS revealed an angle that was slit to closed to the upper TM, consistent with PIS. After treatment with brimonidine and pilocarpine OS, a 360° ALPI OS was performed using settings of 500-μm spot size, 0.7-s-duration, and 240-mW power, with post-procedure reductions in IOP measurement to 18 mmHg OD and 14 mmHg OS. She was switched to brimonidine TID OU and continued on prednisolone QID OS. The following day, her IOP measurements were 20 mmHg OD and 16 mmHg OS. Dark-room gonioscopy revealed open angles OU, with some slit areas OS. A touch-up ALPI OS using settings of 200-μm spot size, 0.5-s-duration, and 280-mW power was performed to eliminate any remaining appositional closure, with repeated dark-room gonioscopy showing open angles for 360° OS.
3. Discussion
Our patient presented with many of the characteristic clinical and radiologic features of NPS, including hypoplastic fingernails with absent lunulae, swan-neck finger deformities, loss of DIP skin creases, patellar hypoplasia, limited elbow range of motion, talipes equinovarus, musculoskeletal pain, hypoplastic lateral humeral epicondyles and radial head dislocation. However, she deviated from previously identified cases in the novel nature of her ocular involvement. OHTN and OAG have been associated with NPS, with POAG as the most commonly reported abnormality.1,14,20,21,23 Pigmentary glaucoma, congenital glaucoma, and normal-tension glaucoma have also been associated with NPS.14,20,21 However, to our knowledge, there have been no previous reports of PIS or ACG in a patient with NPS.
Plateau iris is a common cause of angle-closure in younger patients, predominantly in women 30–50 years of age.25 Plateaus iris configuration (PIC) occurs when the iris root angulates forward and then centrally, thereby narrowing the anterior chamber angle despite a normal range anterior chamber depth. The sine-wave shaped curve of the peripheral iris appears as a double hump sign on indentation gonioscopy. When appositional closure persists after a patent iridotomy has eliminated any contributing pupillary block, this condition is termed plateau iris syndrome.26 PIS can be classified as either complete or incomplete depending on the height of the iris plateau. In complete PIS, angle-closure occurs at the level of the upper trabecular meshwork or Schwalbe's line, resulting in IOP elevations like those seen in our patient, whereas in incomplete PIS, the closure occurs at the mid-meshwork level, leaving the IOP unaffected.27 Patients with PIS can develop extensive PAS, persistently elevated IOPs, and ultimately, ACG.26
In a study of 67 patients aged ≤40 years with angle-closure, Ritch et al. found that 52% had a diagnosis of PIS. Similar to our patient, most of these patients (74%) were female, with a mean age of 34.9 ± 4.6 years at the time of diagnosis.25 Unlike in our patient, a family history of ACG is often seen in this population. In contrast to angle-closure due to relative pupillary block, which more commonly occurs in middle-aged and elderly individuals, angle-closure due to plateau iris tends to occur in younger patients who are less hyperopic, as in this case.25 High hyperopia increases a patient's risk for angle-closure due to relative pupillary block, as hyperopic eyes are anatomically smaller with anterior chambers that are shallow and therefore more prone to crowding.25,28, 29, 30 This is distinct from our young patient with angle-closure due to plateau iris, who was only slightly hyperopic, with a normal-range axial length (21.9 mm OU) and anterior chamber depth (2.7 mm OU).31, 32, 33 Medical management of this condition begins with use of topical miotic drugs (e.g. pilocarpine) that promote contraction of the iris sphincter and ciliary body muscles, thereby thinning the iris and distancing it from the trabecular meshwork. However, LPI and ALPI are the primary treatment modalities for patients with PIC and PIS, respectively, as was the case for our patient.34,35
Currently, there is no established genetic basis for an association between NPS and ACG, as exists for NPS and OAG. It is possible that our patient's presentation is coincidental, rather than an ocular manifestation of her systemic disease. However, recent genome-wide association studies have found that although POAG and primary ACG (PACG) were once thought to be distinct, certain loci (PLEKHA7, FERMT2) previously implicated in PACG are also associated with OAG and IOP.36,37 Given that large-scale genetic studies are now showing subtle but significant genome-wide overlap between IOP, POAG, and PACG, we would not be surprised if there is similar pleiotropy at LMX1B, which is already associated with OAG, with regards to ACG.
Furthermore, LMX1B is expressed in a variety of tissues, notably in the periocular mesenchyme and its derivatives, including the iris, ciliary body, and trabecular meshwork. In a study of the murine eye, Pressman et al demonstrated that in the absence of lmx1b expression, mice that are homozygous for a targeted mutation of lmx1b display anterior segment anomalies, such as iris and ciliary body stromal hypoplasia, lack of ciliary folds, and reduction in anterior chamber depth.10 LMX1B thus plays an integral role in the development of the anatomical structures involved in ACG associated with PIS, which results from iris or ciliary body abnormalities, such as anteriorly positioned ciliary processes.27
Our patient's particular LMX1B genetic variant [c.668G>C (p.Arg223Pro)] is extremely rare. A review of databases containing over 100,000 exomes and over 1,200 LMX1B variants revealed no previous reports of this variant.24,38 As such, the role of this specific variant in the development of PIS or ACG in a patient with NPS has not yet been explored. Given its rarity, further research is necessary to elucidate the functional effect of this particular LMX1B variant and its contributions to the development of PIS and ACG.
4. Conclusion
Clinical and genetic associations between NPS and OAG are well-established. Our patient presented with ACG associated with PIS, which has not been previously described in a patient with NPS, and she possessed a rare LMX1B variant that lacks documentation in the current literature. Her case thus represents a novel genetic and clinical presentation of NPS and the associated ocular manifestations.
Patient consent
The patient consented orally to publication of the case.
Funding
Funded in part by the Sharon Patrick Glaucoma Research Fund of the New York Eye and Ear Infirmary of Mount Sinai, New York, NY. Role limited to financial support for costs of production and publication.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
None.
Acknowledgements
None.
Contributor Information
Margot A. Gardin, Email: gardin.margot@gmail.com.
Chiea Chuen Khor, Email: khorcc@gis.a-star.edu.sg.
Luis Silva, Email: cigof.glaucoma@gmail.com.
Einar A. Krefting, Email: einar.krefting@gmail.com.
Robert Ritch, Email: ritchmd@glaucoma.net.
References
- 1.Sweeney E., Fryer A., Mountford R., Green A., McIntosh I. Nail patella syndrome: a review of the phenotype aided by developmental biology. J Med Genet. 2003;40(3):153–162. doi: 10.1136/jmg.40.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witzgall R. Nail-patella syndrome. Pflugers Arch Eur J Physiol. 2017;469(7-8):927–936. doi: 10.1007/s00424-017-2013-z. [DOI] [PubMed] [Google Scholar]
- 3.Little E.M. Congenital absence or delayed development of the patella. Lancet. 1897;150:781–784. [Google Scholar]
- 4.Bongers E.M.H.F., Gubler M.C., Knoers N.V.A.M. Nail-patella syndrome. Overview on clinical and molecular findings. Pediatr Nephrol. 2002;17(9):703–712. doi: 10.1007/s00467-002-0911-5. [DOI] [PubMed] [Google Scholar]
- 5.McIntosh I., Dreyer S.D., Clough M.V. Mutation analysis of LMX1B gene in Nail-patella syndrome patients. Am J Hum Genet. 1998;63(6):1651–1658. doi: 10.1086/302165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vollrath D., Jaramillo-Babb V.L., Clough M.V. Loss-of-function mutations in the LIM-homeodomain gene, LMX1B, in nail-patella syndrome. Hum Mol Genet. 1998;7(7):1091–1098. doi: 10.1093/hmg/7.7.1091. [DOI] [PubMed] [Google Scholar]
- 7.Riddle R.D., Ensini M., Nelson C., Tsuchida T., Jessell T.M., Tabin C. Induction of the LIM homeobox gene Lmx1 by WNT6a establishes dorsoventral pattern in the vertebrate limb. Cell. 1995;83(4):631–640. doi: 10.1016/0092-8674(95)90103-5. [DOI] [PubMed] [Google Scholar]
- 8.Vogel A., Rodriguez C., Warnken W., Belmonte J.C.I. Dorsal cell fate specified by chick Lmx1 during vertebrate limb development. Nature. 1996;379(6568):848. doi: 10.1038/379848c0. [DOI] [PubMed] [Google Scholar]
- 9.Kania A., Johnson R.L., Jessell T.M., Jesse T.M. Coordinate roles for LIM homeobox genes in directing the dorsoventral trajectory of motor axons in the vertebrate limb. Cell. 2000;102(2):161–173. doi: 10.1016/S0092-8674(00)00022-2. [DOI] [PubMed] [Google Scholar]
- 10.Pressman C.L., Chen H., Johnson R.L. Lmx1b, a LIM homeodomain class transcription factor, is necessary for normal development of multiple tissues in the anterior segment of the murine eye. Genesis. 2000;26(1):15–25. doi: 10.1002/(SICI)1526-968X(200001)26:1<15::AID-GENE5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 11.Morello R., Zhou G., Dreyer S.D. Regulation of glomerular basement membrane collagen expression by LMX1B contributes to renal disease in nail patella syndrome. Nat Genet. 2001;27(2):205–208. doi: 10.1038/84853. [DOI] [PubMed] [Google Scholar]
- 12.Rohr C., Prestel J., Heidet L. The LIM-homeodomain transcription factor Lmx1b plays a crucial role in podocytes. J Clin Invest. 2002;109(8):1073–1082. doi: 10.1172/JCI0213961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miner J.H., Morello R., Andrews K.L. Transcriptional induction of slit diaphragm genes by Lmx1b is required in podocyte differentiation. J Clin Invest. 2002;109(8):1065–1072. doi: 10.1172/JCI0213954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bongers E.M.H.F., Huysmans F.T., Levtchenko E. Genotype-phenotype studies in nail-patella syndrome show that LMX1B mutation location is involved in the risk of developing nephropathy. Eur J Hum Genet. 2005;13(8):935–946. doi: 10.1038/sj.ejhg.5201446. [DOI] [PubMed] [Google Scholar]
- 15.Smidt M.P., Asbreuk C.H.J., Cox J.J., Chen H., Johnson R.L., Burbach J.P.H. A second independent pathway for development of mesencephalic dopaminergic neurons requires Lmx1b. Nat Neurosci. 2000;3(4):337–341. doi: 10.1038/73902. [DOI] [PubMed] [Google Scholar]
- 16.Cheng L., Chen C.L., Luo P. Lmx1b, pet-1, and Nkx2.2 coordinately specify serotonergic neurotransmitter phenotype. J Neurosci. 2003;23(31):9961–9967. doi: 10.1523/jneurosci.23-31-09961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding Y.Q., Marklund U., Yuan W. Lmx1b is essential for the development of serotonergic neurons. Nat Neurosci. 2003;6(9):933–938. doi: 10.1038/nn1104. [DOI] [PubMed] [Google Scholar]
- 18.Dreyer S.D. LMX1B transactivation and expression in nail-patella syndrome. Hum Mol Genet. 2000;9(7):1067–1074. doi: 10.1093/hmg/9.7.1067. [DOI] [PubMed] [Google Scholar]
- 19.Sato U., Kitanaka S., Sekine T., Takahashi S., Ashida A., Igarashi T. Functional characterization of LMX1B mutations associated with nail-patella syndrome. Pediatr Res. 2005;57(6):783–788. doi: 10.1203/01.PDR.0000157674.63621.2C. [DOI] [PubMed] [Google Scholar]
- 20.Mimiwati Z., Mackey D.A., Craig J.E. Nail-patella syndrome and its association with glaucoma: a review of eight families. Br J Ophthalmol. 2006;90(12):1505–1511. doi: 10.1136/bjo.2006.092619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lichter P.R., Richards J.E., Downs C.A., Stringham H.M., Boehnke M., Farley F.A. Cosegregation of open-angle glaucoma and the nail-patella syndrome. Am J Ophthalmol. 1997;124(4):506–515. doi: 10.1016/S0002-9394(14)70866-9. [DOI] [PubMed] [Google Scholar]
- 22.Romero P., Sanhueza F., Lopez P., Reyes L., Herrera L.C. 194 a>C (Q65P) mutation in the LMX1B gene in patients with nail-patella syndrome associated with glaucoma. Mol Vis. 2011;17(July):1929–1939. [PMC free article] [PubMed] [Google Scholar]
- 23.Ghoumid J., Petit F., Holder-Espinasse M. Nail-Patella Syndrome: clinical and molecular data in 55 families raising the hypothesis of a genetic heterogeneity. Eur J Hum Genet. 2016;24(1):44–50. doi: 10.1038/ejhg.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karczewski K.J., Francioli L.C., Tiao G. Variation across 141,456 human exomes and genomes reveals the spectrum of loss-of-function intolerance across human protein-coding genes. bioRxiv. 2019:531210. doi: 10.1101/531210. [DOI] [Google Scholar]
- 25.Ritch R., Chang B.M., Liebmann J.M. Angle closure in younger patients. Ophthalmology. 2003;110(10):1880–1889. doi: 10.1016/S0161-6420(03)00563-3. [DOI] [PubMed] [Google Scholar]
- 26.Wright C., Tawfik M.A., Waisbourd M., Katz L.J. Primary angle-closure glaucoma: an update. Acta Ophthalmol. 2016;94(3):217–225. doi: 10.1111/aos.12784. [DOI] [PubMed] [Google Scholar]
- 27.Ritch R., Dorairaj S. Plateau iris syndrome in younger patients. Clin Exp Ophthalmol. 2007;35(5):399–400. doi: 10.1111/j.1442-9071.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 28.Lowe R.F. Aetiology of the anatomical basis for primary angle-closure glaucoma. Biometrical comparisons between normal eyes and eyes with primary angle-closure glaucoma. Br J Ophthalmol. 1970;54(3):161–169. doi: 10.1136/bjo.54.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Herick W., Shaffer R.N., Schwartz A. Estimation of width of angle of anterior chamber. Incidence and significance of the narrow angle. Am J Ophthalmol. 1969;68:626–629. doi: 10.1016/0002-9394(69)91241-0. [DOI] [PubMed] [Google Scholar]
- 30.Jonas J.B., Aung T., Bourne R.R., Bron A.M., Ritch R., Panda-Jonas S. Glaucoma. Lancet. 2017;390(10108):2183–2193. doi: 10.1016/S0140-6736(17)31469-1. [DOI] [PubMed] [Google Scholar]
- 31.Fontana S.T., Brubaker R.F. Volume and depth of the anterior chamber in the normal aging human eye. Arch Ophthalmol. 1980;98(10):1803–1808. doi: 10.1001/archopht.1980.01020040655013. [DOI] [PubMed] [Google Scholar]
- 32.Congdon N.G., Youlin Q., Quigley H. Biometry and primary angle-closure glaucoma among Chinese, White, and black populations. Ophthalmology. 1997;104(9):1489–1495. doi: 10.1016/S0161-6420(97)30112-2. [DOI] [PubMed] [Google Scholar]
- 33.Oliveira C., Harizman N., Girkin C.A. Axial length and optic disc size in normal eyes. Br J Ophthalmol. 2007;91(1):37–39. doi: 10.1136/bjo.2006.102061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cornel S., Adriana I.D., Mehdi B., Mihaela T.C., Algerino D.S. Plateau iris – diagnosis and treatment. 2015;59(1):14–18. [Google Scholar]
- 35.Ritch R., Tham C.C.Y., Lam D.S.C. Long-term success of argon laser peripheral iridoplasty in the management of plateau iris syndrome. Ophthalmology. 2004;111(1):104–108. doi: 10.1016/j.ophtha.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Khawaja A.P., Cooke Bailey J.N., Wareham N.J. Genome-wide analyses identify 68 new loci associated with intraocular pressure and improve risk prediction for primary open-angle glaucoma. Nat Genet. 2018;50(6):778–782. doi: 10.1038/s41588-018-0126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacGregor S., Ong J.S., An J. Genome-wide association study of intraocular pressure uncovers new pathways to glaucoma. Nat Genet. 2018;50(8):1067–1071. doi: 10.1038/s41588-018-0176-y. [DOI] [PubMed] [Google Scholar]
- 38.Landrum M.J., Lee J.M., Benson M. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018;46(D1):D1062–D1067. doi: 10.1093/nar/gkx1153. [DOI] [PMC free article] [PubMed] [Google Scholar]