Abstract
Objective: Triglycerides (TG) to high density lipoprotein (HDL) ratio has been proposed as a marker of insulin resistance and atherosclerosis. We hypothesize that TG/HDL ratio correlates positively with global cardiac microcalcification as assessed by NaF-PET/CT as a surrogate marker for coronary atherosclerosis in healthy non-diabetic individuals. Method: We identified 68 healthy, non-diabetic individuals (age 41.7 ± 13.5 years; 35/33 female/male) from the CAMONA trial. All underwent PET/CT imaging 90 minutes after NaF injection (2.2 Mbq/Kg). Global cardiac average SUVmean (aSUVmean) was calculated by a trained physician for each individual. Fasting plasma lipid profile (total cholesterol (TC), low-density lipoprotein (LDL), HDL, and TG) and fasting plasma glucose were recorded. TG/HDL ratio was calculated for every individual. Univariate and multivariate linear regression models were used to assess the association between TG/HDL ratio and global cardiac aSUVmean. Result: On univariate analysis, there was a positive linear association of TG/HDL ratio and global cardiac aSUVmean (r=0.244, B=0.047, P=0.045). On multivariate analysis adjusted for age, gender, systolic blood pressure, diastolic blood pressure, smoking status, total cholesterol, low-density lipoprotein, and fasting plasma glucose, TG/HDL ratio was found to be independently associated with global cardiac aSUVmean (B=0.060, 95% CI: 0.007-0.114, P=0.027). Conclusion: There was a positive correlation between TG/HDL ratio with global cardiac microcalcification assessed by NaF-PET/CT imaging.
Keywords: Triglyceride, high density lipoprotein, cardiac microcalcification, NaF, PET/CT
Introduction
Coronary artery disease is a major cause of morbidity and mortality globally and dyslipidemia is a modifiable risk factor [1]. The role of elevated low-density lipoprotein (LDL) cholesterol in the development of atherosclerosis and cardiovascular disease is well recognized and is the primary target of preventive therapy [2]. LDL particles are heterogeneous in nature and vary in their size, density, composition, and atherogenic potential. Amongst them, small, dense LDL (sdLDL) is a highly atherogenic particle that is associated with a significantly elevated risk for coronary artery disease [3-6]. However, their level is not routinely measured despite its strong association with cardiovascular disease. Triglyceride (TG) to high-density lipoprotein (HDL) ratio has been proposed as a surrogate lipid marker for sdLDL [7]. Also, TG/HDL ratio has been reported as a marker of insulin resistance [8]. Thus TG/HDL ratio represents as easy to measure parameter to identify individuals with an increased cardio-metabolic risk.
The association of TG/HLD ratio and the burden of coronary atherosclerosis in asymptomatic non-diabetic individuals has not been studied. 18F-Sodium Fluoride PET is a novel non-invasive imaging technique that has been used to visualize atherosclerosis. 18F-Sodium Fluoride selectively deposits in microcalcifications and can identify early stage atherosclerotic lesions even before it’s detected on CT imaging [9]. In this study, we aimed to examine the correlation of TG/HDL ratio and the extent of subclinical coronary atherosclerosis in non-diabetic individuals using 18F-Sodium Fluoride PET/CT, hypothesizing that an increasing TG/HDL ratio correlates positively with increasing global uptake of this radiotracer.
Methods
This study was conducted in a subset of asymptomatic subjects from the prospective study known as “Cardiovascular Molecular Calcification Assessed by 18F-FDG PET/CT (CAMONA)” in Odense, Denmark. The CAMONA study was approved by the Danish National Committee on Biomedical Research Ethics as well as registered at ClinicalTrials.gov (NCT01724749) [10]. The study was undertaken in concordance with the Declaration of Helsinki and all subjects provided written informed consent. The subjects in this population were excluded based on the presence of malignancy, immunodeficiency syndrome, autoimmune disease, pregnancy, sarcoidosis, amyloidosis, endocarditis, symptoms suggestive of cardiovascular disease such as syncope, chest pain, and shortness of breath, as well as use of prescription medications.
In this cohort, we analyzed 68 asymptomatic community-dwelling non-diabetic individuals from the CAMONA trial. Fasting plasma lipid profile (Total cholesterol, High-density lipoprotein, Low-density lipoprotein, and Triglyceride) and fasting plasma glucose were measured. TG/HDL ratio was measured for each individual by dividing fasting triglyceride by high-density lipoprotein level.
Quantitative image analysis
All subjects in the study cohort underwent NaF PET/CT imaging with an established and uniform protocol (GE Discovery STE, VCT, RX, and 690/710). Patients were made to observe an overnight fast of 6 hours and a blood glucose measurement ensuring a concentration below 8 mmol/L. These individuals underwent PET/CT imaging 90 minutes after the injection of NaF (2.2 Mbq/Kg). These images were produced using one of several PET/CT systems (GE Discovery STE, VCT, RX, and 690/710). PET images were corrected for scanner dead time, attenuation, scatter, and random coincidences. CT imaging (140 kV, 30-110 mA, noise index 25, 0.8 seconds per rotation, slice thickness 3.75 mm) was done to account for attenuation correction and anatomic referencing with PET scans.
The analysis was performed on axial images using OsiriX MD software. The global cardiac uptake of NaF uptake (Global cardiac aSUVmean) was measured by a trained physician using a DICOM viewer (Osirix MD Software; Pixmeo, SARL, Bernex, Switzerland) to generate regions of interest (ROI) (Figure 1). It was done by manually defining regions of interest on each axial slice excluding the cardiac valves, aortic wall, and nearby skeletal structures. The application to utilize the global assessment method to investigate atherosclerotic microcalcification in the coronary arteries with Na-F has been established in previous studies [11-13]. For every ROI, which represents the volume of one cardiac slice, the NaF activity was determined as the mean standardize uptake value. These values were then added and divided by the sum of the ROI-defined slice volumes to yield a global cardiac average mean standardize uptake value (aSUVmean) for each patient.
Figure 1.
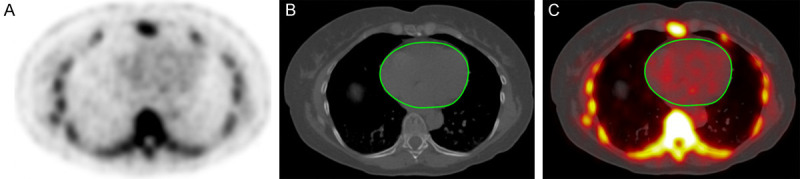
Axial NaF-PET (A), CT (B), and fused NaF-PET/CT with region of interest (C) in a 62-year-old individual. The manually-delineated region of interest determined the global coronary artery NaF uptake and did not include uptake from the aortic valve, skeletal structures, and aortic wall.
Statistical analysis
The association between TG/HDL ratio to Global cardiac aSUVmean was determined. This was done using univariate and multivariate regression models adjusting for common atherosclerotic risk factors (age, gender, systolic blood pressure, diastolic blood pressure, smoking status, total cholesterol, low-density lipoprotein, and fasting plasma glucose) were employed to assess the association between TG/HDL ratio and Global cardiac aSUVmean. A p value <0.05 was determined to be statistically significant. We used statistical software packages SPSS (Version 25.0, IBM) and STATA/MP 16.1 (StataCorp, College Station, Texas 77845 USA) for the statistical analysis.
Results
Baseline characteristics
The mean (± SD) age in the cohort was 41.7 ± 13.5 years, and included 35 females and 33 males. The mean (± SD) TC, LDL, HDL, TG, fasting plasma glucose and TG/HDL ratio in the cohort were 190 ± 33.5 mg/dl, 117.7 ± 31.3 mg/dl, 56.4 ± 18.5 mg/dl, 91.7 ± 57.9 mg/dl, 98.6 ± 8.7 mg/dl and 1.95 ± 1.91 respectively. In this cohort, mean (± SD) SBP was 126.8 ± 14.6, DBP was 75.6 ± 9.4, and 25% were smokers. The average Framingham risk score was less than 10% in the overall study population (Table 1).
Table 1.
Subject demographics
| Characteristics | Mean ± SD |
|---|---|
| Age | 41.7 ± 13.5 |
| Male (%) | 48.5 |
| Systolic blood pressure (mm Hg) | 126.8 ± 14.6 |
| Diastolic blood pressure (mm Hg) | 75.6 ± 9.4 |
| TG:HDL ratio (umol/L) | 1.95 ± 1.91 |
| Low density lipoprotein (mg/dl) | 117.7 ± 31.3 |
| Total cholesterol (mg/dl) | 190 ± 33.5 |
| Triglycerides (mg/dl) | 91.7 ± 57.9 |
| HDL cholesterol (mg/dl) | 56.4 ± 18.5 |
| Fasting plasma glucose (mg/dl) | 98.6 ± 8.7 |
| Smoking (%) | 25 |
| 10-year Framingham Risk Score | 4.95 ± 4.93 |
Note: HbA1c = Glycated hemoglobin. N=20.
Regression analysis
On univariate analysis, there was a positive linear association of TG/HDL ratio and global cardiac aSUVmean (R=0.244, B=0.047, P=0.045) (Figure 2). On multivariate regression analysis after adjusting for age, gender, systolic blood pressure, diastolic blood pressure, smoking status, total cholesterol, low-density lipoprotein, and fasting plasma glucose, showed TG/HDL ratio was independently associated with global cardiac aSUVmean (Table 2).
Figure 2.
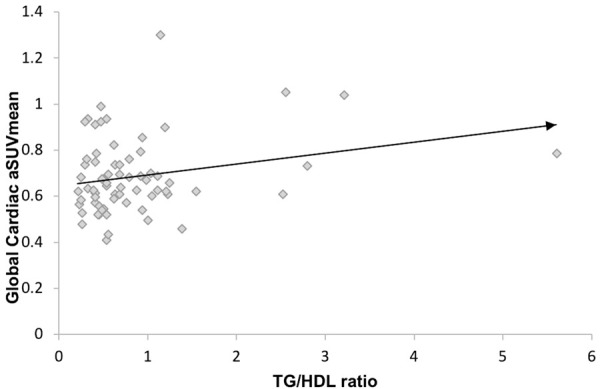
Regression analysis of Global Cardiac aSUVmean and TG/HDL ratio in asymptomatic non-diabetic individuals; R=0.244, P=0.045.
Table 2.
Multivariable linear regression
| Variable | Coefficient (B) | 95% CI | P-value |
|---|---|---|---|
| TG/HDL Ratio | 0.060 | 0.007-0.114 | 0.027 |
| Age | 0.001 | -0.003-0.004 | 0.588 |
| Gender | 0.035 | -0.060-0.130 | 0.459 |
| Total Cholesterol | 0.025 | -0.089-0.139 | 0.660 |
| LDL Cholesterol | -0.023 | -0.142-0.095 | 0.649 |
| Average Systolic BP | -0.001 | -0.005-0.003 | 0.625 |
| Average Diastolic BP | 0.001 | -0.005-0.007 | 0.739 |
| Fasting Plasma Glucose | 0.013 | -0.079-0.105 | 0.775 |
| Smoking | -0.082 | -0.177-0.014 | 0.092 |
Discussion
Vascular calcification is one of the major hallmarks in the paradigm for atherosclerosis pathogenesis. In the early stages of atherosclerotic plaque progression deposition of calcium phosphate microcrystals occur within the necrotic core and surrounding extracellular matrix rich in collagen [9]. Molecular imaging with 18F-NaF PET/CT allows for this microcalcification to be detected non-invasively, thus facilitating earlier identification of atherosclerosis much before its detection via macroscopic calcification on computed tomography or ultimately through the clinical manifestation of the underlying disease, e.g. myocardial infarction [14]. Identification of factors associated with atherosclerosis is an important step in the process of developing strategies to mitigate this disease. In this study, we utilized 18F-NaF PET/CT for in-vivo detection of coronary atherosclerosis and found an independent and positive correlation between global coronary NaF uptake with TG/HDL ratio in asymptomatic non-diabetic individuals. To our knowledge, this is the first study examining the relationship between subclinical coronary atherosclerosis and TG/HDL ratio using 18F-NaF PET imaging technology.
Dyslipidemia, in particular elevated plasma LDL, is a very well-known risk factor and has been causally associated with coronary artery disease [15]. Prior studies have reported that a “high triglyceride and low HDL” or “elevated TG/HDL ratio” state to be associated with coronary atherosclerosis [16-19]. This elevated risk is related to mechanisms involving a complex interplay between TG rich lipoprotein metabolism and vascular inflammation. Elevation in TG rich VLDL (Very Low-Density Lipoprotein) concentrations results in the generation of sdLDL via hepatic lipase. Small dense LDL cholesterol has a longer plasma half-life due to decreased binding to LDL receptors on hepatocytes, is more readily oxidized, and can easily enter the vascular intima compared to larger LDL particles. Elevated TG rich VLDL levels also increase the catabolism of HDL, a key cardio-protective lipoprotein involved in reverse cholesterol transport. Besides, apo-CIII containing TG rich VLDL and free fatty acids released from lipolysis of TG exhibit pro-inflammatory properties. A high TG/HDL ratio also represents a surrogate for an insulin-resistant state which further promotes vascular inflammation, thus accelerating atherosclerosis [20].
Prior studies have reported an elevated TG/HDL ratio correlated with the extent of coronary artery disease in high-risk patient populations undergoing invasive coronary angiography [17-19]. In the study population analyzed by da Luz et al., nearly one third had diabetes mellitus and three quarters had hypertension (blood pressure >140/90) [19]. Similar to prior studies, our findings showed a greater burden of subclinical coronary atherosclerosis with increasing TG/HDL ratios as evidenced by greater uptake of NaF. However, our study population was significantly younger, had no diabetes or hypertension, and overall had a low risk for adverse cardiovascular outcomes. We believe our novel findings would add valuable information to both imaging and atherosclerosis literature. More importantly, it underscores the need for further investigation into the role of non-traditional atherosclerotic risk factors and primary preventive strategies addressing subclinical atherosclerosis.
Our study has several limitations. First, this was a retrospective, cross-sectional, single-center study and included a relatively small sample size with all individuals belonging to a single race (Caucasian). This raises the possibility of selection bias and generalizability of the study findings. Therefore future studies are needed to validate these findings with larger and racially diverse sample sizes. Second, the present study did not examine the relationship between the findings of 18F-NaF PET/CT and angiographic data, either via coronary CT angiogram or invasive diagnostic coronary angiography, thus making it is difficult to assess the degree of coronary artery stenosis in individuals with high NaF uptake or elevated TG/HDL ratio. Third, due to the cross-sectional nature of the study design, we are not able to report the causal relationship between TG/HDL ratio and subclinical atherosclerosis, as well as, prospective clinical outcomes associated the findings such as cardiovascular death, myocardial infarction or angina allowing for the development of risk stratifying tools.
Increasing TG/HDL ratios are associated with an increasing burden of global subclinical coronary atherosclerosis in asymptomatic non-diabetic individuals as assessed by 18F NaF-PET/CT. Because of adverse outcomes associated with subclinical coronary atherosclerosis, including death, earlier identification with NaF-PET/CT and therapeutic interventions among individuals with elevated TG/HDL ratios could potentially save lives.
Acknowledgements
The Jørgen and Gisela Thrane’s Philanthropic Research Foundation, Broager, Denmark, financially supported the CAMONA study.
Disclosure of conflict of interest
None.
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. 2019;139:e56–528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary. J Am Coll Cardiol. 2019;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Arai H, Kokubo Y, Watanabe M, Sawamura T, Ito Y, Minagawa A, Okamura T, Miyamato Y. Small dense low-density lipoproteins cholesterol can predict incident cardiovascular disease in an urban Japanese cohort: the suita study. J Atheroscler Thromb. 2013;20:195–203. doi: 10.5551/jat.14936. [DOI] [PubMed] [Google Scholar]
- 4.Krauss RM. Lipoprotein subfractions and cardiovascular disease risk. Curr Opin Lipidol. 2010;21:305–311. doi: 10.1097/MOL.0b013e32833b7756. [DOI] [PubMed] [Google Scholar]
- 5.Lamarche B, St-Pierre AC, Ruel IL, Cantin B, Dagenais GR, Despres JP. A prospective, population-based study of low density lipoprotein particle size as a risk factor for ischemic heart disease in men. Can J Cardiol. 2001;17:859–865. [PubMed] [Google Scholar]
- 6.Schaefer EJ, Wilson PW, Cupples LA, White CC, Nakajima K, Ito Y, Asztalos BF, Otokozawa S, Ai M. Small dense LDL cholesterol and coronary heart disease: results from the Framingham offspring study. Clin Chem. 2010;56:967–976. doi: 10.1373/clinchem.2009.137489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobiás-ová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate inapob-lipoprotein-depleted plasma (FERHDL) Clin Biochem. 2001;34:583–588. doi: 10.1016/s0009-9120(01)00263-6. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Chavez A, Simental-Mendia LE, Elizondo-Argueta S. Elevated triglycerides/HDL-cholesterol ratio associated with insulin resistance. Cir Cir. 2011;79:126–131. [PubMed] [Google Scholar]
- 9.Shioi A, Ikari Y. Plaque calcification during atherosclerosis progression and regression. J Atheroscler Thromb. 2018;25:294–303. doi: 10.5551/jat.RV17020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blomberg BA, de Jong PA, Thomassen A, Lam MGE, Vach W, Olsen MH, Mali WPTM, Narula J, Alavi A, Høilund-Carlsen PF. Thoracic aorta calcification but not inflammation is associated with increased cardiovascular disease risk: results of the CAMONA study. Eur J Nucl Med Mol Imaging. 2017;44:249–258. doi: 10.1007/s00259-016-3552-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blomberg BA, Thomassen A, de Jong PA, Lam MGE, Diederichsen ACP, Olsen MH, Mickley H, Mali WPTM, Alavi A, Høilund-Carlsen PF. Coronary fluorine-18-sodium fluoride uptake is increased in healthy adults with an unfavorable cardiovascular risk profile: results from the CAMONA study. Nucl Med Commun. 2017;38:1007–1014. doi: 10.1097/MNM.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 12.Moghbel M, Al-Zaghal A, Werner TJ, Constantinescu CM, Hoilund-Carlsen PF, Alavi A. The role of PET in evaluating atherosclerosis: a critical review. Semin Nucl Med. 2018;48:488–497. doi: 10.1053/j.semnuclmed.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Sorci O, Batzdorf AS, Mayer M, Rhodes S, Peng M, Jankelovits AR, Hornyak JN, Gerke O, Hoilund-Carlsen PF, Alavi A, Rajapakse CS. (18)F-sodium fluoride PET/CT provides prognostic clarity compared to calcium and Framingham risk scoring when addressing whole-heart arterial calcification. Eur J Nucl Med Mol Imaging. 2020;47:1678–1687. doi: 10.1007/s00259-019-04590-3. [DOI] [PubMed] [Google Scholar]
- 14.Høilund-Carlsen PF, Sturek M, Alavi A, Gerke O. Atherosclerosis imaging with 18F-sodium fluoride PET: state-of-the-art review. Eur J Nucl Med Mol I. 2019;47:1538–1551. doi: 10.1007/s00259-019-04603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White J, Swerdlow DI, Preiss D, Fairhurst-Hunter Z, Keating BJ, Asselbergs FW, Sattar N, Humphries SE, Hingorani AD, Holmes MV. Association of lipid fractions with risks for coronary artery disease and diabetes. JAMA Cardiol. 2016;1:692–699. doi: 10.1001/jamacardio.2016.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bittner V, Johnson BD, Zineh I, Rogers WJ, Vido D, Marroquin OC, Bairey-Merz CN, Sopko G. The triglyceride/high-density lipoprotein cholesterol ratio predicts all-cause mortality in women with suspected myocardial ischemia. Am Heart J. 2009;157:548–555. doi: 10.1016/j.ahj.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobiášová M, Frohlich J. Fractional esterification rate of cholesterol and ratio of triglycerides to HDL-cholesterol are powerful predictors of positive findings on coronary angiography. Clin Chem. 2003;49:1873–1880. doi: 10.1373/clinchem.2003.022558. [DOI] [PubMed] [Google Scholar]
- 18.Drexel H, Aczel S, Marte T, Benzer W, Langer P, Moll W, Saely CH. Is atherosclerosis in diabetes and impaired fasting glucose driven by elevated LDL cholesterol or by decreased HDL cholesterol? Diabetes Care. 2004;28:101–107. doi: 10.2337/diacare.28.1.101. [DOI] [PubMed] [Google Scholar]
- 19.da Luz PL, Favarato D, Faria-Neto JR Jr, Lemos P, Chagas AC. High ratio of triglycerides to hdl-cholesterol predicts extensive coronary disease. Clinics. 2008;63:427–432. doi: 10.1590/S1807-59322008000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welty FK. How Do Elevated Triglycerides and low HDL-cholesterol affect inflammation and atherothrombosis? Curr Cardiol Rep. 2013;15:400. doi: 10.1007/s11886-013-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


