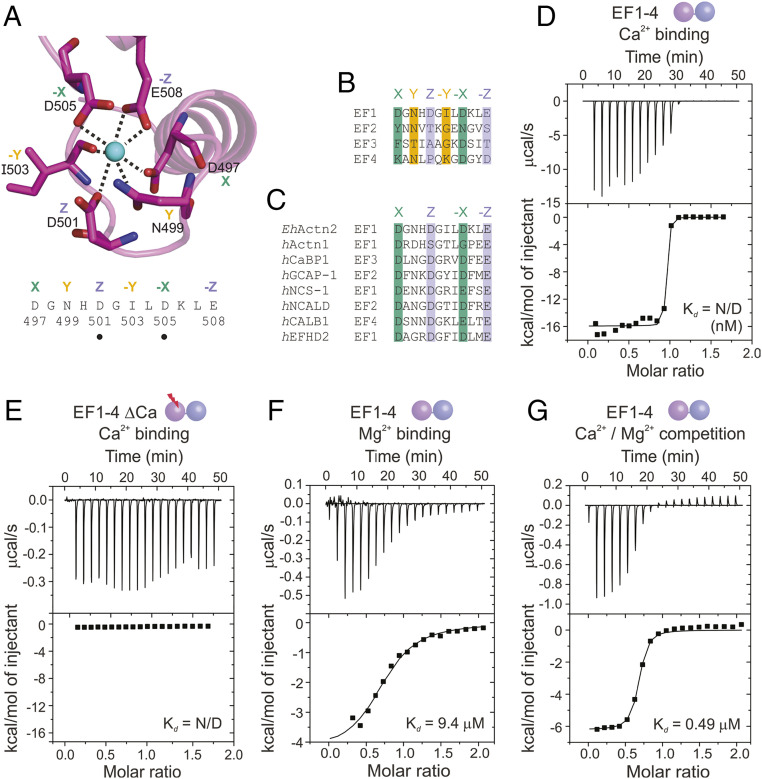
Fig. 2.
EhActn2 shows exceptionally high affinity for Ca2+ and is regulated by Ca2+ in the presence of Mg2+. (A) Structure of the Ca2+-binding loop of EhActn2 EF1-2 shown in a ribbon representation. Residues involved in the Ca2+-binding coordination sphere are shown in green (X, −X axes), orange (Y, −Y axes), and violet (Z, −Z axes). Positions of generated mutants are indicated with a dot. (B) Sequence alignment of potential Ca2+-binding loops in EhActn2 EF hands. Same color code as in A. (C) Sequence alignment of Ca2+-binding loops for EhActn2 EF1 and other calcium-binding proteins with nanomolar Ca2+ affinity. hActn1, human α-actinin-1 (UniProt [UP] code P12814); hCaBP1, human calcium-binding protein 1 (UP code Q9NZU7); hGCAP-1, human guanylyl cyclase-activating protein 1 (UP code P43080); hNCS-1, human neuronal calcium sensor 1 (UP code P62166); hNCALD, human neurocalcin-delta (UP code P61601); hCALB1, human calbindin (UP code P05937); hEFHD2, human EF-hand domain-containing protein D2 (UP code Q96C19). Color-coded as in A. (D–G) ITC profiles of Ca2+ binding to EF1-4 (D); Ca2+ binding to EF1-4 ΔCa (E); Mg2+ binding to EF1-4 (F); and Ca2+ binding to EF1-4 in the presence of Mg2+ (G). Determined Kd values are indicated. N/D, not determined.