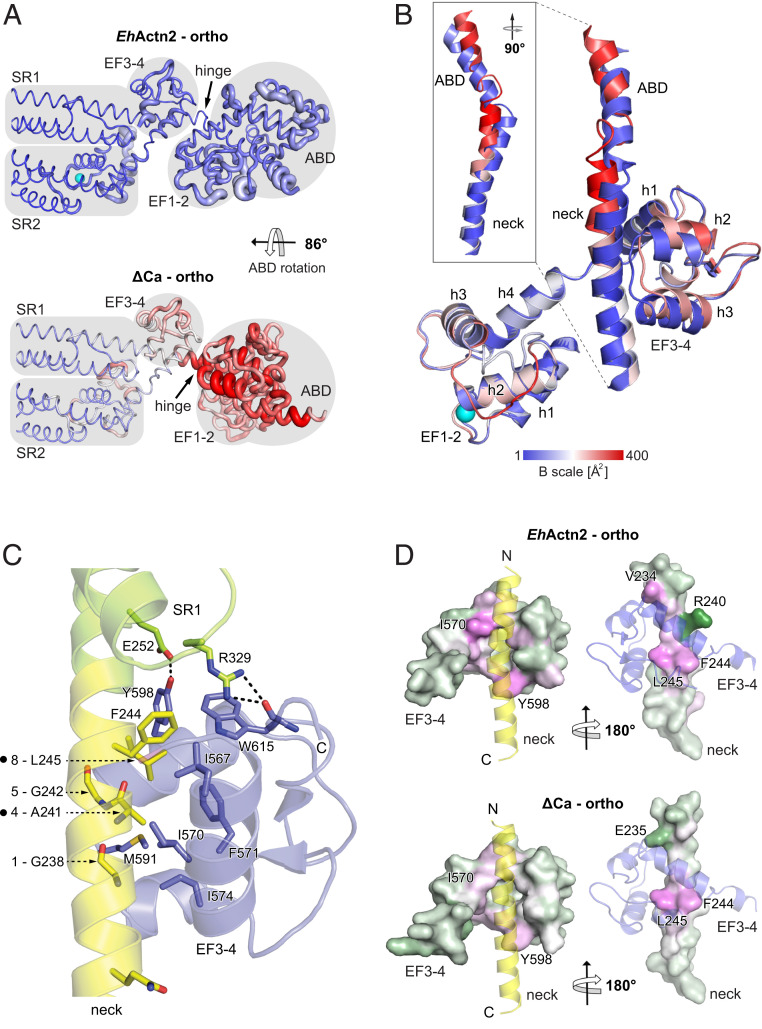
Fig. 3.
Ca2+ binding stabilizes the EhActn2 structure. (A) B-factor representation of Ca2+-bound and Ca2+-free (ΔCa) EhActn2 structures crystallized in the same orthorhombic space group. The color code from blue to red shows the absolute B factors, coil thickness represents the relative difference in B factors, and domain boundaries are shaded in gray. (B) Superposition of EF1-2 from Ca2+-bound EhActn2 and ΔCa showing the conformational differences in the neck and EF3-4 (plus a part of the ABD C-terminal α-helix). The structures are colored according to absolute B factors as in A. (C) Ribbon representation of the major polar and hydrophobic interactions between the neck region (yellow) and EF3-4 (blue). Positions of generated mutants are indicated with a dot next to the neck 1-4-5-8 motif. (D) Surface representation of the neck and EF3-4 from Ca2+-bound EhActn2 and ΔCa colored based on residue solvation energy contributions (green corresponds to negative contribution, white to neutral, and magenta to positive). A positive value makes a negative contribution to the solvation energy gain of the interface, corresponding to a hydrophobic effect. Two orientations rotated by 180° are shown, in which the interacting domain is represented as a transparent ribbon.