Significance
Electrochemical water splitting to generate hydrogen and oxygen is proposed as a promising way for building a clean-energy future. One of the challenges facing this scenario is to develop robust electrocatalysts, especially for the kinetically sluggish oxygen evolution reaction (OER) process. In this work, the delafossite analog was proposed by mutation of metal oxyhydroxides and delafossite. We have experimentally realized the delafossite analog by intercalation of Ag into cobalt–iron (oxyhydr)oxide layers via electrochemical self-reconstruction (ECSR). The delafossite analog exhibits enhanced OER activity and outstanding stability. Our findings demonstrate that in situ ECSR can be an effective approach to construct new types of catalysts. Our advances on delafossite analogs pave the vital way for a class of promising electrochemical catalysts.
Keywords: delafossite analog, electrochemical self-reconstruction, oxygen evolution reaction, amorphization, enhanced activity
Abstract
Development of novel and robust oxygen evolution reaction (OER) catalysts with well-modulated atomic and electronic structure remains a challenge. Compared to the well-known metal hydroxides or (oxyhydr)oxides with lamellar structure, delafossites (ABO2) are characterized by alternating layers of A cations and edge-sharing BO2 octahedra, but are rarely used in OER due to their poor electron conductivity and intrinsic activity. Here, we propose a delafossite analog by mutation of metal oxyhydroxide and delafossite based on first-principles calculations. Modulation on the electronic structure due to distortion of the original crystal field of the BO2 layers is calculated to enhance electron conductivity and catalytic activity. Inspired by the theoretical design, we have experimentally realized the delafossite analog by electrochemical self-reconstruction (ECSR). Operando X-ray absorption spectroscopy and other experimental techniques reveal the formation of delafossite analog with Ag intercalated into bimetallic cobalt–iron (oxyhydr)oxide layers from a metastable precursor through amorphization. Benefitting from the featured local electronic and geometric structures, the delafossite analog shows superior OER activity, affording a current density of 10 mA⋅cm−2 at an overpotential of 187 mV and an excellent stability (300 h) in alkaline conditions.
Electrochemical water splitting is a promising, environmentally friendly alternative to generate clean fuels (1, 2). Despite great efforts in the field, the presently available approaches to electrochemical water splitting are not fit to be upscaled and serve the public, due to their kinetically sluggish reactive processes. This is especially true for the oxidative half-reaction: the oxygen evolution reaction (OER), which used to rely on highly active, but scarce, noble metal compounds as electrocatalysts (3, 4). To overcome this problem, various kinds of efficient and low-cost OER catalysts with distinctive structures (e.g., lamellar, two-dimensional, metal-organic framework, and perovskite) have been developed (5–10). Among these, lamellar structures have received great attention, as they can provide open spaces, highly accessible active sites, and tunable electronic states and properties. Transition metal hydroxides or (oxyhydr)oxide and their derivatives with characteristic MO2 octahedral layers have been widely investigated and applied in the OER field (8, 11–13). Nevertheless, delafossites (ABO2), made of two-dimensional layers with the edge-linked BO2 octahedra connected by O–A+–O dumbbells, have been seldom used in electrocatalysis, due to their poor conductivity and intrinsic activity (14–16). AgBO2 delafossites (e.g., AgFeO2 and AgCoO2) show low OER catalytic activity, despite containing Fe or Co (17, 18). However, delafossites still present an appealing structure for electrochemical catalysis due to their large BO2 interlayer spacing and the strong covalent interaction between Ag and BO2 layers. This consideration motivates further fundamental research in developing delafossite analogs with electronic properties suitable for electrocatalytic applications.
Although research in delafossites is mature, the exploration of delafossite analogs and relevant syntheses is underdeveloped (14). Thus, while desirable, discovery of viable synthesis pathways for delafossite analogs remains a great challenge. Recently, some catalytic materials have been observed to be labile to application of an external potential, and thereby to convert into new substances responsible for the actual OER activity (19, 20). Fabbri et al. (21) reported that Ba0.5Sr0.5Co0.8Fe0.2O3-δ nano-powders could undergo self-reconstruction during the OER process with formation of a bimetal oxy(hydroxide) active layer in a high-oxidation state. Similarly, electrochemically induced structural transformations were also discovered for NiFe Prussian blue analogs and CoOx systems (22, 23). Though these phenomena have been observed and extended our mechanistic understanding of OER activity, to date, little attention has been paid to employing self-reconstruction in electrochemical process as a strategy to produce new substances or phases with enhanced electrocatalytic activity.
To this end, here, we designed and fabricated the delafossite analog through mutation of the atomic structure of delafossite and metal oxyhydroxide by virtue of both density functional theory (DFT) calculations and in situ electrochemical self-reconstruction (ECSR) in experiment. The delafossite analog not only inherits the layered structure from both the delafossite and oxyhydroxide, but it also exhibits a flexible electronic structure, which can be easily modulated to enhance the OER activity. To overcome the difficulty of intercalating metal ions into (oxyhydr)oxide layers, we rely on in situ ECSR of a metastable precursor. Several in situ and ex situ experimental techniques confirm intercalation of Ag in the interlayer regions of the (oxyhydr)oxide. The ensuing electrode shows a low overpotential of ∼187 mV at 10 mA⋅cm−2 and long-time stability (300 h) for OER in alkaline conditions.
Results
Theoretical Calculation.
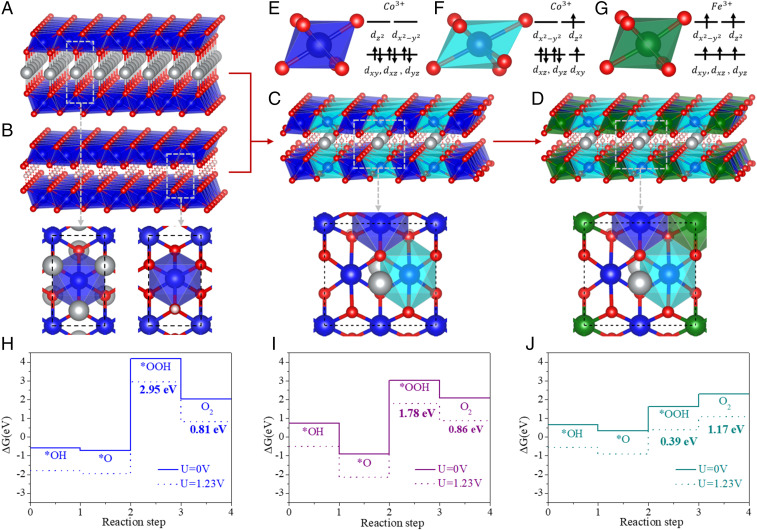
Fig. 1A displays the atomic configuration of AgCoO2 with a typical delafossite structure, which consists of alternating layers of close-packed Ag cations and edge-sharing CoO2 octahedra. Conversely, CoOOH merely contains edge-sharing CoO2 octahedral layers (Fig. 1B). It is generally understood that electron conductivity is crucial to sustain electrocatalytic activity. Thus, we performed first-principles calculations to understand the electronic structures of the CoO2 layers. For pure CoOOH and AgCoO2, the Co atoms stayed in the center of octahedral crystal field with high symmetries. The corresponding dxy, dxz, and dyz states were occupied, while the dz2 and dz2-y2 ones were empty (Fig. 1E and SI Appendix, Fig. S1A). Considering t2g was occupied and the eg was empty in Co3+ for either CoOOH or AgCoO2, the energy differences between t2g and eg orbitals corresponded to the calculated energy gaps of 1.98 eV for CoOOH and 1.24 eV for AgCoO2 (SI Appendix, Fig. S2 A and B), respectively. The existence of a band gap should be the main reason for the poor conductivity of CoOOH and AgCoO2 (15).
Fig. 1.
Scheme of delafossite analog of AxBO2H1-x (A, Ag; B, Co and Fe; x, 1/4) based on the mutation of the delafossite and oxyhydroxide and their OER activity. (A–D) The atomic structures (side view) of different configurations: AgCoO2 (A), CoOOH (B), AgxCoO2H1-x (x = 1/4) (C), and AgxFeyCo1-yO2H1-x (x = 1/4, y = 1/4) (D), and the top views of the atomic structures are shown below the panels. (E–G) The electronic configurations and the corresponding crystal fields: low-spin Co3+ (E), high-spin Co3+ (F), and high-spin Fe3+ (G). (H–J) The calculated ∆G of the OER: CoOOH (H), Ag1-xCoO2Hx (I), and AgxFeyCo1-yO2H1-x (J). Here, the white, red, blue, green, and silver balls represent H, O, Co, Fe, and Ag atoms, respectively. The dark blue, sky blue, and green polygons stand for the octahedral crystal fields of the original Co–O, stretched Co–O, and Fe–O, respectively.
Although CoOOH and AgCoO2 presented similar crystal fields and quite close structures, the bonding feature and interlayer distance between layers were different. The mutation of these two compounds may effectively modulate the electronic structure. The specific scheme of combining the CoOOH and AgCoO2 is shown in Fig. 1 A–C. The different compositions were considered from AgCoO2 to AxBO2H1-x (A, Ag; B, Co; x, 1/4) (Fig. 1C), by reducing the ratio of Ag from 1 to 1/4. During this process, the H atoms remained the feature of CoOOH, while the Ag in new compound stayed in the triangular prism of six oxygen atoms, rather than in the linear O–Ag–O configurations, as in AgCoO2. Notably, the Co–O bonds closest to the Ag were stretched from 1.94 to 2.14 Å, which slightly distorted the symmetry of the octahedral crystal field of Co3+. The Co3+ electronic configuration changed from t2g6eg0 into t2g5eg1 (Fig. 1F and SI Appendix, Fig. S1B). In addition, the hybrid functional calculations with HSE06 also confirmed that the electronic configuration of Co3+ nearby the Ag became t2g5eg1 instead of t2g6eg0. As discussed above, with half of the Co3+ ions in the new AxBO2H1-x (A, Ag; B, Co; x, 1/4) compound behaving as t2g5eg1, the band gap was reduced to 0.60 eV (SI Appendix, Fig. S2C). Thus, the high-spin Co3+ induced by Ag atom can largely change the electronic structure and effectively enhance the conductivity.
To further modulate the electronic structure for high conductivity, some Co atoms were further substituted by Fe atoms, and the composition of AxBO2H1-x (A, Ag; B, Co and Fe; x, 1/4) was constructed (Fig. 1D). The Fe atom in Ag1/4Co3/4Fe1/4O2H3/4 preferred the high-spin state of t2g3eg2 instead of the low-spin state of t2g5eg0 (Fig. 1G and SI Appendix, Fig. S1C). Both the dz2 and dx2-y2 states of Fe3+ were half-occupied. The high-spin Fe atoms in distorted CoO2 layers completely eliminated the band gap, turning the material from a semiconductor into a metal (SI Appendix, Fig. S2D). Thus, Ag1/4Co3/4Fe1/4O2H3/4 should exhibit high conductivity, which is favorable to electrochemical OER catalysis.
The metal–O bonding mainly affected the formation and releasing of O2 in the OER process by extracting the electrons from the substrate; thus, the delafossite analog with the Fe and Ag atoms may improve the OER activity. To this end, the free energies (∆G) of the OER process on these different compounds were investigated by using first-principles calculations. The corresponding overpotential (η) for each system was examined (Fig. 1 H–J; and see calculation details in SI Appendix). The η for pure CoOOH was 2.95 V (Fig. 1H), which decreased to 1.78 V Ag1/4CoO2H3/4 (Fig. 1I) and further down to 1.17 V for Ag1/4Co3/4Fe1/4O2H3/4 (Fig. 1J). Thus, the Fe and Ag atoms can lower the OER overpotential of the pure CoOOH because of the change of electronic structure. With the modulating of electronic structure from semiconductor to metal and the effective reduction of η in OER, the delafossite analog is predicted to exhibit high OER activity.
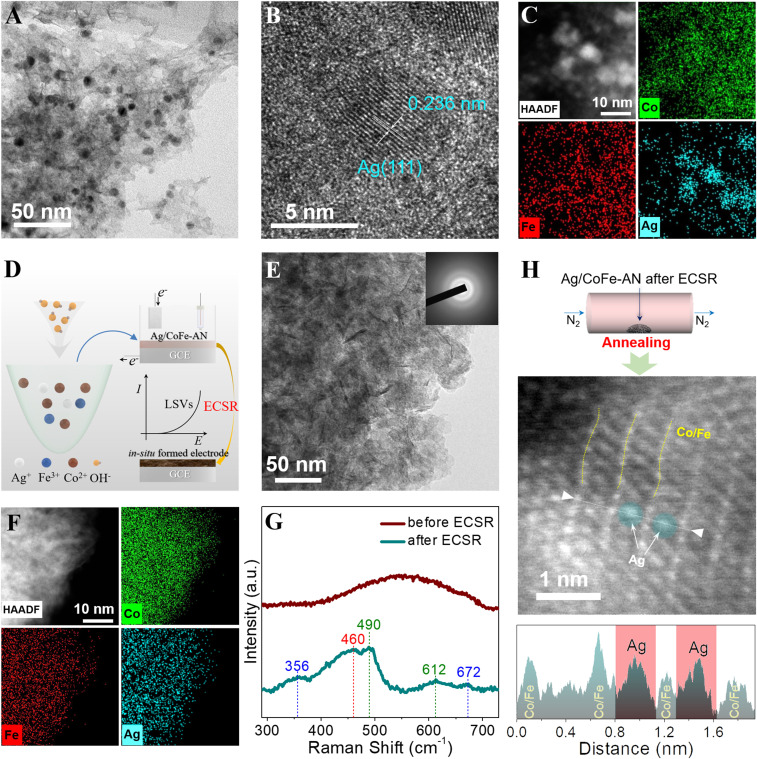
Motivated by the DFT results, we turned to fabricate the delafossite analog by means of ECSR. We firstly synthesized Ag nanoparticles attached on cobalt–iron hydr(oxy)oxide amorphous nanosheets (Ag/CoFe-AN) as the precursor for the targeted electrode by quickly dropping alkaline solution into the alcoholic nitrate solution of cobaltous, ferric, and silver (see details in SI Appendix). As shown in Fig. 2A, the transmission electron microscopy (TEM) image of Ag/CoFe-AN shows a crumpled and entangled sheet structure, decorated with nanoparticles of 5- to 10-nm diameters (see more details in SI Appendix, Fig. S3). X-ray diffraction (XRD) analysis showed that only characteristic peaks of Ag nanocrystals existed, which disclosed that the cobalt–iron hydr(oxy)oxide nanosheets are in the amorphous state (SI Appendix, Fig. S4). The lattice fringes of the nanocrystals disclosed by high-resolution TEM (HRTEM) are consistent with the Ag crystal structure (Fig. 2B). Element mapping in Fig. 2C demonstrates the uniform distribution of Co and Fe and clustered distribution of Ag, suggesting a homogeneous alloying structure of cobalt-ion hydr(oxy)oxide nanosheets with nanoparticle Ag. Based on the inductively coupled plasma optical emission spectrometry analysis, the molar ratio of Co:Fe:Ag is determined to be 100:23:19, which is in accordance with the energy-dispersion spectrum (EDS) analysis (SI Appendix, Fig. S5).
Fig. 2.
Ex situ analysis on Ag/CoFe-AN before and after ECSR. (A) The TEM image of Ag/CoFe-AN. (B) The HRTEM image of Ag/CoFe-AN. The lattice spacing of 0.236 nm is assigned to the (111) planes of Ag. (C) HAADF-STEM image of Ag/CoFe-AN and related EDS mapping images. (D) The schematic illustration of the formation of Ag/CoFe-AN and the in situ catalyst. (E) Postmortem TEM image of Ag/CoFe-AN after ECSR. E, Inset reports the corresponding SAED image. (F) Postmortem HAADF-STEM image and related EDS mapping images. (G) Raman spectra for Ag/CoFe-AN before and after ECSR. (H) The HAADF-STEM image of annealed Ag/CoFe-AN after ECSR and the corresponding HAADF intensity line profiles. a.u., arbitrary units.
The ECSR of Ag/CoFe-AN was carried out in an electrochemical activation procedure by simple linear-sweep voltammetries (LSVs) in a three-electrode system before OER performance evaluation (Fig. 2D and SI Appendix, Fig. S6). To identify the possible change of Ag/CoFe-AN after ECSR, we investigated its postmortem morphology. As shown in Fig. 2E, the Ag nanoparticles disappeared after ECSR, and only stacked nanosheets were left. The selected area electron diffraction (SAED) image demonstrated that the postmortem electrode is in an amorphous state, which is consistent with the XRD pattern (SI Appendix, Fig. S7). The high-angle annular dark-field scanning TEM (HAADF-STEM) image and EDS mapping (Fig. 2F) revealed that Co, Fe, and Ag were uniformly distributed in the sample. These characterizations clearly indicate that the initial Ag nanoparticles have degraded during the process, and a multielement homodispersed phase was finally formed, maintaining the pristine atomic ratio (SI Appendix, Fig. S8).
The disappearance of the Ag nanoparticles can also be confirmed by ultraviolet-visible (UV-vis) absorption spectra (SI Appendix, Fig. S9). The adsorption peak at around 410 nm clearly indicated the existence of Ag nanoparticles in Ag/CoFe-AN before ECSR, which vanished after the ECSR process (24). We also employed X-ray photoelectron spectroscopy (XPS) to detect the electronic state of the Ag element. In SI Appendix, Fig. S10, the peaks at 368.4 and 367.8 eV can be assigned to the presence of Ag0 and Ag+, respectively, indicating the oxidation of Ag in electrochemical process (25). Raman spectra were carried out to investigate the structural change of Ag/CoFe-AN, as shown in Fig. 2G. Notably, several peaks emerged in the electrode material after ECSR, in contrast to the one before ECSR with only a bump around 400 to 700 cm−1. Of these, the two characteristic peaks appeared at 490 and 612 cm−1, which can be assigned to cobalt oxyhydroxide species (26, 27). The peak at 460 cm−1 can be attributed to CoO2 in a hexagonal delafossite structure (28). The residual peaks at 356 and 672 cm−1 are associated with the bending and stretching vibrations of the Fe–O in an octahedral environment of silver ferrite delafossite species (29).
In order to know the atomic configuration of Ag/CoFe-AN after ECSR, we annealed it into crystalline (SI Appendix, Fig. S11), which was further characterized by HAADF-STEM. As shown in Fig. 2H, the highlighted spots with the large radius correspond to Ag atoms, staying in the lattice fringes of Co/Fe atoms (30). It is worth noting that the intercalation of Ag atoms into the interlayers hardly occurs during the solvent-free annealing process (31, 32). Accordingly, the postmortem electrode is conjectured to be a delafossite analog, with Ag atoms staying in the interlayers of cobalt–iron (oxyhydr)oxide.
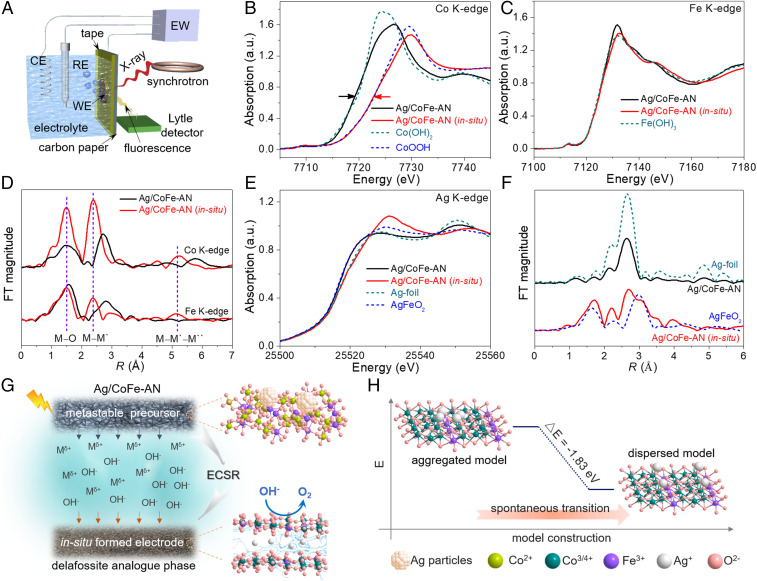
To better understand the evolution process and catalytic centers of the electrode, in situ X-ray absorption spectroscopy was used to monitor the operando behavior of our electrode. The home-built electrochemical cell and the integral setup for the operando experiment are shown in Fig. 3A. As shown in Fig. 3B, the position of the Co K edge of Ag/CoFe-AN is approximate to that of Co(OH)2 in X-ray absorption near-edge spectroscopy (XANES). In comparison, the Co absorption edge of Ag/CoFe-AN in the in situ state was substantially shifted to higher energies, almost overlapping with that of CoOOH. This result demonstrates the oxidation of the Co2+ sites to higher-valence cobalt species (i.e., cobalt oxyhydroxide species). Conversely, no apparent shift was observed for the absorption edge of Fe in pristine and in situ state of Ag/CoFe-AN (Fig. 3C), indicating that the valence of Fe was almost unchanged.
Fig. 3.
In situ XAS characterization of initial Ag/CoFe-AN and its self-reconstruction into delafossite analog during ECSR. (A) Schematic illustration of the in situ XAS experimental setup, where EW, CE, RE, and WE stand for electrochemical workstation, counter electrode, reference electrode, and working electrode, respectively. (B and C) XANES data for Co and Fe K-edges of Ag/CoFe-AN collected on the initial state and in situ state and reference materials Co(OH)2, CoOOH, and Fe(OH)3. (D) Co and Fe K-edge FT-EXAFS, and M′ and M″ represent near metal sites (Co, Fe). (E and F) XANES data (E) and FT-EXAFS (F) for Ag K-edge of Ag/CoFe-AN collected on the initial state and in situ state together with counterparts. Here, the in situ experiments were performed during the potentiostatic OER process at potential of 1.46 V vs. RHE after ECSR, and the catalyst in the in situ state is marked as Ag/CoFe-AN (in situ). (G) Self-reconstruction process from the precursor (upper) to the delafossite analog (lower) by ECSR. (H) The relative energy and atomic models of aggregated Ag cluster (upper) and the dispersed Ag atoms (lower) on the cobalt–iron (oxyhydr)oxide layer. a.u., arbitrary units.
The Fourier-transformed k3-weighted extended X-ray absorption fine structure (FT-EXAFS) spectra further displayed that the bond distances of Co–O, Co–M′, Fe–O, and Fe–M′ self-evidently decreased, revealing the change in coordination environment of the Co and Fe (Fig. 3D). The in situ Co and Fe K-edge FT-EXAFS demonstrated similar M–O and M–M′ peak positions. Meanwhile, apparent peaks appeared at about double the nearest M–M′ distances, which mainly originated from multiple-scattering in collinear M–M′–M″ arrangements. These results clearly indicate that the Fe substitution of Co occurred within sheets, rather than intercalation into the CoO2 interlayer (33).
Remarkable changes can also be observed for Ag in XANES spectra in Fig. 3E, indicating that the local structure of Ag atoms was changed. The changes of the electronic structure and local atomic environment of Ag can be captured by the k3-weighted Ag K-edge FT-EXAFS spectra (Fig. 3F). The FT-EXAFS data of Ag in initial Ag/CoFe-AN shows two peaks located at ∼2.2 and 2.6 Å, corresponding to the Ag–Ag coordination in Ag metal. At in situ state, an additional peak developed at ∼1.7 Å, which is a typical scattering feature of the Ag–O bond, demonstrating that Ag atoms were oxidized during the electrochemical process (34). The bond distance of Ag–O was identified to be 2.12 Å (SI Appendix, Fig. S12 and Table S1), consistent with the first-principles results of 2.11 Å, but larger than that of Ag2O (2.07 Å) or AgFeO2 (2.08 Å). In addition, the Ag–Ag and Ag–Co/Fe with bond distance of 2.89 and 3.53 Å also indicate the change of Ag coordination environment from initial Ag–Ag in Ag nanoparticles to analogous configuration of AgFeO2.
Here, we integrate information to prove the formation of a delafossite analog. Firstly, the complete oxidation of zerovalent Ag nanoparticles after ECSR was proved by comprehensive characterizations (Figs. 2 E and F and 3F and SI Appendix, Figs. S7, S9, and S10). The oxidation product of Ag should not be Ag2O or other AgOx species in higher-valence states due to their longer bond distance of Ag–O and apparent Ag–Ag and Ag–Co/Fe coordinations for Ag/CoFe-AN (in situ), as well as XPS analysis of the postmortem electrode (SI Appendix, Table S1 and Figs. S10 and S12) (35, 36). As to the location of Ag atoms, the possibilities that Ag substitutes Co/Fe in octahedra or anchors on the surface of the cobalt–iron (oxyhydr)oxide layers like single-atom supporting can be excluded from the substantial difference in ionic charge and radius and high content of Ag (SI Appendix, Fig. S8). Here, because of the complete oxidation of Ag, the Ag–Ag coordination in Ag/CoFe-AN (in situ) should be attributed to the interlayer monovalent Ag, similar to delafossite rather than metal Ag particles. Meanwhile, the typical Ag–Co/Fe coordination also strongly suggests that the spatial location relationship of Ag and Co/Fe is analogous to that of AgFeO2 with delafossite structure. Thus, the presented coordination of Ag–O, Ag–Ag, and Ag–Co/Fe should derive from interlayer Ag among cobalt–iron (oxyhydr)oxide layers. Along with the Raman and HAADF-STEM analysis, we were to conclude that the ECSR had induced a delafossite analog with Ag intercalated into bimetallic (oxyhydr)oxide layers.
This dramatic change in phase structure during ECSR can be attributed to potential-driven self-reconstruction—that is, a dynamic “dissolution–redeposition” process (Fig. 3G). The self-reconstruction generally involves several steps: 1) applying potential; 2) the degradation and cations dissolution of the precursor material triggered by the oxidation of lattice oxygen; 3) the subsequent redeposition of leaching cations reacting with OH−; and 4) the formation of a new phase (21, 37–39). In parallel, besides atom motion at the solid–liquid interface, atom migration and ion exchange can also occur in the solid-phase interior (40–43). As shown in Fig. 3H, the first-principles calculations show that the aggregated Ag cluster on the cobalt–iron (oxyhydr)oxide layer is about 1.83 eV less stable than the dispersed one, which should be the driving force for the homogenization of Ag. Thus, this process is expected to introduce pronounced atom rearrangement. The nanoscale Ag particles and amorphous sheet matrix endow Ag/CoFe-AN with metastable characteristic and a large solid–liquid interface; thus, the redox and dissolution of metal sites, atomic migration, and ion exchange become favorable, enabling a facile potential-induced self-reconstruction (44, 45).
Notably, we also developed several typical counterparts, including Co&Fe and Ag&Co bimetal compounds (CoFe–AN and Ag/Co–AN) and a Co monometal compound (Co–AN), and all of them demonstrated apparent structural transformation in in situ catalysis, which indicated that the self-reconstruction of these metastable precursors should be a general method for new phase (SI Appendix, Figs. S13–S18). In contrast, crystalline bulk phases after annealing Ag/CoFe-AN at 600 °C (Ag/CoFe-CB) showed insignificant change of absorption edge at an applied potential, indicating that the rigid crystalline structure retards the reconstruction of the material (SI Appendix, Figs. S19 and S20). Thus, the initial metastable precursor material plays the vital role in self-reconstruction.
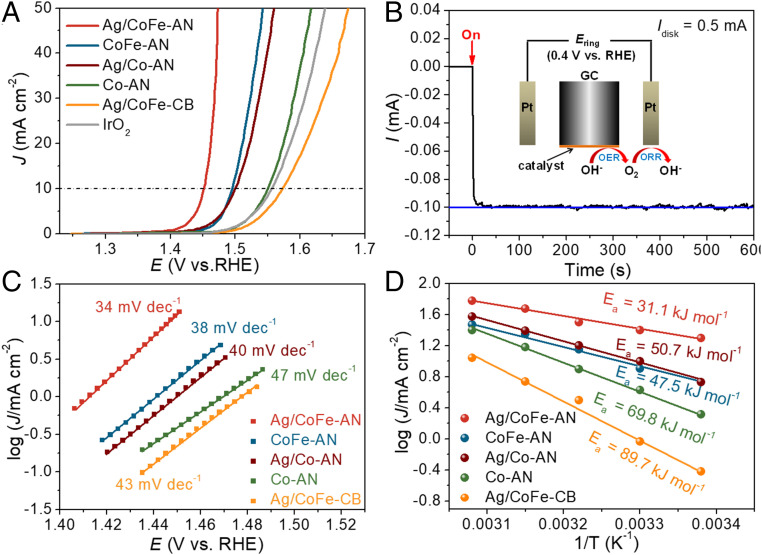
We tested the electrocatalytic OER properties of the Ag/CoFe-AN electrode along with the counterparts. All catalytic materials were characterized by the same electrochemical activation procedure and evaluated in a 1 M KOH electrolyte solution by using a three-electrode configuration. Fig. 4A shows the anodic polarization currents recorded by LSVs of all of the six electrodes. The overpotential at the current density of 10 mA⋅cm−2, η(10 mA⋅cm−2), is a crucial benchmark to determine the catalytic activity of materials (46). Remarkably, Ag/CoFe-AN exhibited the lowest η(10 mA⋅cm−2) value of 220 mV in comparison with the counterparts (SI Appendix, Table S2). We also tested the activity of Ag/CoFe-AN on a more conductive electrode substrate of nickel foam plated with gold. The Ag/CoFe-AN shows an even lower η(10 mA⋅cm−2) value of 187 mV (SI Appendix, Fig. S21) (47).
Fig. 4.
Electrochemical performance of Ag/CoFe-AN. (A) The iR-corrected LSVs on a glass carbon electrode. (B) Faraday efficiency testing of Ag/CoFe-AN using rotating-ring disk electrode measurements (schematic shown as Inset) in N2-saturated 1 M KOH. The O2 generated from Ag/CoFe-AN at a constant current of 0.5 mA was reduced at the Pt ring at a constant potential of 0.4 V vs. RHE. (C) Tafel plots. (D) Arrhenius plot of the kinetic current at ƞ = 0.27 V, tested on high-temperature-resistance glassy carbon electrode.
The turnover frequency and mass activity of Ag/CoFe-AN at overpotential of 270 mV reached 0.535 s−1 and 2,028.60 A⋅g−1, which are much higher than those of other catalytic materials, as shown in SI Appendix, Table S2, demonstrating its high intrinsic activity. The measured Faradaic efficiency is as high as 100 ± 1% in Fig. 4B, proving that the observed catalytic current originates exclusively from water oxidation. As shown in Fig. 4C, the Tafel slope of our Ag/CoFe-AN is 34 mV⋅dec−1 smaller than that of others, indicating its superior reaction kinetics (9). The activation energy (Ea) of the OER can be extracted from the slope of the Arrhenius plot. In comparison, Ag/CoFe-AN exhibits the minimum Ea value of 31.1 kJ⋅mol−1, which is suggestive of its lowest kinetic barrier and high catalysis capacity (Fig. 4D) (48). The electrochemically active surface area (ECSA) of catalytic materials and corresponding mass-normalized ECSA (ECSAm) were estimated from double-layer capacitance (Cdl), which was obtained by cyclic voltammetry cycles in the range of no Faradaic processes (SI Appendix, Fig. S22 and Table S2) (49, 50). Apparently, Ag/CoFe-AN has the largest ECSA of 87.7 cm2 and ECSAm of 438.5 m2⋅g−1, which implies that it should possess more accessible active sites. The impressive ECSA of Ag/CoFe-AN should be derived from its unusual analogous delafossite architecture and amorphous structure with abundant interlayer gaps and vacant sites (e.g., ion vacancies and void spaces, etc.), which allow diffusion of electrolytes, even into the inner parts of catalyst matrices, and provide larger solid–liquid interface, as well as electrochemically available areas (51, 52). We normalized current by ECSA (JECSA); Ag/CoFe-AN also delivers the lowest ƞ of 290 mV at JECSA = 0.3 mA⋅cm−2, suggesting its high specific activity (53).
The conductivity of our electrode was tested by using electrochemical impedance spectroscopies shown in SI Appendix, Fig. S23, and the corresponding circuit model-fitting analysis was also performed (SI Appendix, Table S3). The charge–transfer resistance of Ag/CoFe-AN was apparently lower than other counterparts, demonstrating an enhanced electrical conductivity by multimetal compositing. It is noteworthy that Ag/CoFe-AN is one of the best-performance 3d-metal–based catalytic materials and comparable to the state-of-the-art catalysts for OER (SI Appendix, Table S4). The long-time chronopotentiometry was carried out at a current density of 40 mA⋅cm−2 for about 300 h to evaluate the stability of Ag/CoFe-AN. As shown in SI Appendix, Fig. S24, the working potential remained almost constant for the entire testing period, demonstrating its excellent electrochemical durability. Thus, the delafossite analog produced by the ECSR exhibits the superior activity and the long stability for OER, which should benefit from both the special atomic and electronic structures.
Discussion
In summary, we successfully designed and achieved a delafossite analog by both first-principles calculations and in situ ECSR experiments. The first-principles calculations suggest that the delafossite analog integrates atomic and electronic structures favorable to the OER, derived from metal oxyhydroxide and delafossites. To synthesize the predicted delafossite analog, we developed a simple strategy of in situ ECSR to realize Ag intercalation into bimetallic (oxyhydr)oxide layers from a metastable precursor, free of painstaking synthesis procedure. Significantly, the delafossite analog electrode showed superior electrocatalytic activity and stability for the OER. Our work presents the promising prospects of delafossite analogs as materials for electrocatalysis. Successful realization of the proposed in situ ECSR strategy paves avenues for designing and developing advanced catalysts.
Materials and Methods
Synthesis of Ag/CoFe-AN.
In a typical procedure, 21.75 mg of Co(NO3)2·6H2O, 7.56 mg of Fe(NO3)3·9H2O, and 2.55 mg of AgNO3 were added to 60 mL of ethanol under constant stirring in a water bath at 15 °C. And then, 10 mg of NaOH were loaded into a jar containing 1 mL of distilled water and 9 mL of ethanol to form a transparent solution after stirring for several minutes. Subsequently, the NaOH solution was quickly added to the multimetal salt solution, and brown-black precipitation was quickly formed. After 15 min of stirring, the sample was washed by mixture solution of deionized water and ethanol at ratio of 1:5 three times.
Synthesis of CoFe-AN, Ag/Co-AN, and Co-AN.
As references, for preparation of CoFe-AN, Ag/Co-AN, and Co-AN, the same process was followed, except that AgNO3, Fe(NO3)3·9H2O, and both AgNO3 and Fe(NO3)3·9H2O were added, respectively.
Synthesis of Ag/CoFe-CB.
Ag/CoFe-CB was prepared by heat treatment of Ag/CoFe-AN at 600 °C for 3 h in air.
Annealed Postmortem Electrode Material.
We annealed the postmortem electrode material at 250 °C for 3 h in N2.
Details of other experiments, reagents and characterizations, and electrochemical measurements, as well as DFT theoretical calculations, are provided in SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by National Natural Science Foundation of China Grants 51532001, 51861130360, 11974037, and U1930402; and China Postdoctoral Science Foundation Grant 2019TQ0013. L.-M.L. was supported by Newton Advanced Fellowship Grant NAFR1180242. We thank the staff at Beijing and Shanghai Synchrotron Radiation Facility at 1W1B and BL14W1 for providing the beam time and helpful discussion.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2009180117/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Faunce T. A. et al., Energy and environment policy case for a global project on artificial photosynthesis. Energy Environ. Sci. 6, 695–698 (2013). [Google Scholar]
- 2.Walter M. G. et al., Solar water splitting cells. Chem. Rev. 110, 6446–6473 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Suen N.-T. et al., Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 46, 337–365 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Montoya J. H. et al., Materials for solar fuels and chemicals. Nat. Mater. 16, 70–81 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Kim J. S., Kim B., Kim H., Kang K., Recent progress on multimetal oxide catalysts for the oxygen evolution reaction. Adv. Energy Mater. 8, 1702774 (2018). [Google Scholar]
- 6.Faber M. S., Jin S., Earth-abundant inorganic electrocatalysts and their nanostructures for energy conversion applications. Energy Environ. Sci. 7, 3519–3542 (2014). [Google Scholar]
- 7.Xiong B., Chen L., Shi J., Anion-containing noble-metal-free bifunctional electrocatalysts for overall water splitting. ACS Catal. 8, 3688–3707 (2018). [Google Scholar]
- 8.Huang J. et al., CoOOH nanosheets with high mass activity for water oxidation. Angew. Chem. Int. Ed. Engl. 54, 8722–8727 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Zhao S. et al., Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy 1, 16184 (2016). [Google Scholar]
- 10.Kim J., Yin X., Tsao K. C., Fang S., Yang H., Ca2Mn2O5 as oxygen-deficient perovskite electrocatalyst for oxygen evolution reaction. J. Am. Chem. Soc. 136, 14646–14649 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Yang Y. et al., Highly active trimetallic NiFeCr layered double hydroxide electrocatalysts for oxygen evolution reaction. Adv. Energy Mater. 8, 1703189 (2018). [Google Scholar]
- 12.Yin H., Tang Z., Ultrathin two-dimensional layered metal hydroxides: An emerging platform for advanced catalysis, energy conversion and storage. Chem. Soc. Rev. 45, 4873–4891 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Gong M., Dai H., A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res. 8, 23–39 (2015). [Google Scholar]
- 14.Sheets W. C., Mugnier E., Barnabe A., Marks T. J., Poeppelmeier K. R., Hydrothermal synthesis of delafossite-type oxides. Chem. Mater. 18, 7–20 (2006). [Google Scholar]
- 15.Kang J.-S. et al., Photoemission study of AgTO2 delafossites (T = Fe, Co, Ni). Phys. Rev. B Condens. Matter Mater. Phys. 61, 10682–10687 (1999). [Google Scholar]
- 16.Carcia P. F., Shannon R. D., Bierstedt P. E., Flippen R. B., O2 electrocatalysis on thin film metallic oxide electrodes with the delafossite structure. J. Electrochem. Soc. 127, 1974–1978 (1976). [Google Scholar]
- 17.Zhang R. et al., Increase of Co 3D projected electronic density of states in AgCoO2 enabled an efficient electrocatalyst toward oxygen evolution reaction. Nano Energy 57, 753–760 (2019). [Google Scholar]
- 18.Toyoda K. et al., Surface analysis of topmost layer of epitaxial layered oxide thin film: Application to delafossite oxide for oxygen evolution reaction. Surf. Sci. 668, 93–99 (2018). [Google Scholar]
- 19.Li Y. et al., Recent progress on surface reconstruction of earth-abundant electrocatalysts for water oxidation. Small 15, e1901980 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Sivanantham A., Ganesan P., Vinu A., Shanmugam S., Surface activation and reconstruction of non-oxide-based catalysts through in-situ electrochemical tuning for oxygen evolution reactions in alkaline media. ACS Catal. 10, 463–493 (2020). [Google Scholar]
- 21.Fabbri E. et al., Dynamic surface self-reconstruction is the key of highly active perovskite nano-electrocatalysts for water splitting. Nat. Mater. 16, 925–931 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Su X. et al., Operando spectroscopic identification of active sites in NiFe Prussian blue analogues as electrocatalysts: Activation of oxygen atoms for oxygen evolution reaction. J. Am. Chem. Soc. 140, 11286–11292 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Favaro M. et al., Understanding the oxygen evolution reaction mechanism on CoOx using operando ambient-pressure X-ray photoelectron spectroscopy. J. Am. Chem. Soc. 139, 8960–8970 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Zhang F.-J. et al., Surface plasmon resonance induced reduction of high quality Ag/graphene composite at water/toluene phase for reduction of H2O2. Appl. Surf. Sci. 265, 578–584 (2013). [Google Scholar]
- 25.Ferraria A. M., Carapeto A. P., Rego A. M. B. d., X-ray photoelectron spectroscopy: Silver salts revisited. Vacuum 86, 1988–1991 (2012). [Google Scholar]
- 26.Liu Y. C., Koza J. A., Switzer J. A., Conversion of electrodeposited Co(OH)2 to CoOOH and Co3O4, and comparison of their catalytic activity for the oxygen evolution reaction. Electrochim. Acta 140, 359–365 (2014). [Google Scholar]
- 27.Pauporté T., Mendoza L., Cassir M., Bernard M. C., Chivot J., Direct low-temperature deposition of crystallized CoOOH films by potentiostatic electrolysis. J. Electrochem. Soc. 152, C49–C53 (2005). [Google Scholar]
- 28.Chen Z. et al., Reversible structural evolution of NiCoOxHy during the oxygen evolution reaction and identification of the catalytically active phase. ACS Catal. 8, 1238–1247 (2018). [Google Scholar]
- 29.Nagarajan R., Tomar N., Ultrasound assisted ambient temperature synthesis of ternary oxide AgMO2 (M=Fe, Ga). J. Solid State Chem. 182, 1283–1290 (2009). [Google Scholar]
- 30.Krehula S., Music S., Formation of AgFeO2, α-FeOOH, and Ag2O from mixed Fe(NO3)3-AgNO3 solutions at high pH. J. Mol. Struct. 1044, 221–230 (2013). [Google Scholar]
- 31.Balikçi F., Yüceer S. B., Güldür Ç., Comparative study of Ag2O/Co3O4 catalysts prepared by the sol-gel and Co-precipitation techniques: Characterization and CO activity studies. Chem. Eng. Commun. 196, 171–181 (2009). [Google Scholar]
- 32.Dey S., Dhal G. C., Mohan D., Prasad R., Synthesis and characterization of AgCoO2 catalyst for the oxidation of CO at a low temperature. Polyhedron 155, 102–113 (2018). [Google Scholar]
- 33.Friebel D. et al., Identification of highly active Fe sites in (Ni,Fe)OOH for electrocatalytic water splitting. J. Am. Chem. Soc. 137, 1305–1313 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Matsuoka M., Anpo M., Photoluminescence properties and photocatalytic reactivities of Cu+/zeolite and Ag+/zeolite catalysts prepared by the ion-exchange method. Curr. Opin. Solid State Mater. Sci. 7, 451–459 (2003). [Google Scholar]
- 35.Mansour A. N., Evidence for an Ag4O3 phase of silver oxide. J. Phys. Chem. 94, 1006–1010 (1990). [Google Scholar]
- 36.Firet N. J. et al., Operando EXAFS study reveals presence of oxygen in oxide-derived silver catalysts for electrochemical CO2 reduction. J. Mater. Chem. A Mater. Energy Sustain. 7, 2597–2607 (2019). [Google Scholar]
- 37.Yang C. et al., Revealing pH-dependent activities and surface instabilities for Ni-based electrocatalysts during the oxygen evolution reaction. ACS Energy Lett. 3, 2884–2890 (2018). [Google Scholar]
- 38.Zhang R. et al., Dissolution/precipitation equilibrium on the surface of iridium-based perovskites as oxygen evolution reaction catalysts in acidic media. Angew. Chem. Int. Ed. 58, 1–6 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Jiang H. et al., Tracking structural self-reconstruction and identifying true active sites toward cobalt oxychloride precatalyst of oxygen evolution reaction. Adv. Mater. 31, e1805127 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Pi Y., Shao Q., Zhu X., Huang X., Dynamic structure evolution of composition segregated iridium-nickel rhombic dodecahedra towards efficient oxygen evolution electrocatalysis. ACS Nano 12, 7371–7379 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Grimaud A. et al., Activation of surface oxygen sites on an iridium-based model catalyst for the oxygen evolution reaction. Nat. Energy 2, 16189 (2016). [Google Scholar]
- 42.Shin Y. J., Doumerc J. P., Dordor P., Pouchard M., Hagenmuller P., Preparation and physical properties of the delafossite-type solid solutions AgCoxNi1-xO2 (0 ≤ x≤ 0.5). J. Solid State Chem. 107, 194–200 (1992). [Google Scholar]
- 43.Feng X., Liu X., Yuan Y., Fabrication and characterization of AgCoO2 delafossite by cation exchange process. Key Eng. Mater. 602, 11–14 (2014). [Google Scholar]
- 44.Li H. B. et al., Amorphous nickel hydroxide nanospheres with ultrahigh capacitance and energy density as electrochemical pseudocapacitor materials. Nat. Commun. 4, 1894 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chae O. B. et al., Reversible lithium storage at highly populated vacant sites in an amorphous vanadium pentoxide electrode. Chem. Mater. 26, 5874–5881 (2014). [Google Scholar]
- 46.Song F., Hu X., Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis. Nat. Commun. 5, 4477 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Zhang B. et al., Homogeneously dispersed multimetal oxygen-evolving catalysts. Science 352, 333–337 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Zheng X. et al., Theory-driven design of high-valence metal sites for water oxidation confirmed using in situ soft X-ray absorption. Nat. Chem. 10, 149–154 (2018). [DOI] [PubMed] [Google Scholar]
- 49.McCrory C. C. L., Jung S., Peters J. C., Jaramillo T. F., Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 135, 16977–16987 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Song F., Hu X., Ultrathin cobalt-manganese layered double hydroxide is an efficient oxygen evolution catalyst. J. Am. Chem. Soc. 136, 16481–16484 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Durham J. L., Kirshenbaum K., Takeuchi E. S., Marschilok A. C., Takeuchi K. J., Synthetic control of composition and crystallite size of silver ferrite composites: Profound electrochemistry impacts. Chem. Commun. (Camb.) 51, 5120–5123 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Anantharaj S., Noda S., Amorphous catalysts and electrochemical water splitting: An untold story of harmony. Small 16, e1905779 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Jung S., McCrory C. C. L., Ferrer I. M., Peters J. C., Jaramillo T. F., Benchmarking nanoparticulate metal oxide electrocatalysts for the alkaline water oxidation reaction. J. Mater. Chem. A Mater. Energy Sustain. 4, 3068–3076 (2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.