Abstract
Background
Cytomegalovirus (CMV) seropositivity and anti-CMV immunoglobulin G (IgG) levels are associated with adverse health outcomes in elderly populations. Among people living with human immunodeficiency virus (PLWH), CMV seropositivity has been associated with persistent CD8 T-cell elevation and increased risk of developing non-AIDS comorbidities despite long-term antiretroviral therapy (ART). Herein, we investigated whether CMV seropositivity and elevation of anti-CMV IgG levels were associated with increased epithelial gut damage, microbial translocation, and systemic inflammation.
Methods
A total of 150 PLWH (79 ART-naive and 71 ART-treated) were compared to 26 without human immunodeficiency virus (HIV) infection (uninfected controls). Plasma markers of HIV disease progression, epithelial gut damage, microbial translocation, nonspecific B-cell activation, anti-CMV and anti–Epstein-Barr virus (EBV) IgG levels, and proinflammatory cytokines were measured.
Results
CMV seropositivity and elevated anti-CMV IgG levels were associated with markers of epithelial gut damage, microbial translocation, and inflammation in PLWH and participants without HIV infection. In contrast, total nonspecific IgG, immunoglobulin M, immunoglobulin A, and anti-EBV IgG levels were not associated with these markers. CMV seropositivity was associated with markers of epithelial gut damage, microbial translocation, and inflammation independent of sociodemographic and behavioral characteristics of the study population.
Conclusions
CMV-seropositive people with and without HIV had increased epithelial gut damage, microbial translocation, and inflammation. Furthermore, anti-CMV IgG levels were independently associated with increased epithelial gut damage and microbial translocation. CMV coinfection may partially explain persistent gut damage, microbial translocation, and inflammation in ART-treated PLWH.
Keywords: HIV, cytomegalovirus, epithelial gut damage, microbial translocation, inflammation
Cytomegalovirus (CMV) seropositivity and anti-CMV immunoglobulin G levels are associated with increased epithelial gut damage, microbial translocation, and inflammation in antiretroviral therapy (ART)–naive and ART-treated people living with human immunodeficiency virus and uninfected controls.
Human cytomegalovirus (CMV), a member of the Herpesviridae family, is ubiquitous worldwide. In elderly populations, CMV-specific T-cell response and elevated anti-CMV immunoglobulin G (IgG) levels have been linked with adverse health outcomes in large epidemiological studies [1, 2]. The underlying mechanism has yet to be defined but studies showed that a high frequency of CMV-specific T cells skews the immune system toward a CMV-specific response instead of fighting other pathogens [3]. Initial infection occurs primarily in mucosal epithelial cells including the gastrointestinal tract [4]. Recently, CMV has been shown to actively replicate in enterocytes, leading to a loss of gut barrier integrity [5].
Human immunodeficiency virus (HIV) infection is characterized by a rapid decline of mucosal CD4 T cells, impaired gut barrier integrity, and subsequent translocation of microbial products leading to persistent inflammation. Such inflammation contributes to the increased risk of developing non-AIDS comorbidities among people living with HIV (PLWH) receiving antiretroviral therapy (ART) [6]. In a simian immunodeficiency virus–infected rhesus macaque model, Hensley-McBain et al showed that intestinal damage precedes mucosal immune dysfunction and subsequent inflammation [7]. We and others have shown that bacterial and fungal translocation are associated with systemic inflammation and increased risk of developing non-AIDS comorbidities in both ART-naive and ART-treated PLWH [8–12]. As such, understanding the factors associated with persistent epithelial gut damage and inflammation in PLWH is of critical importance.
CMV is a common coinfection among PLWH, and invasive CMV disease has become rare during the ART era [13]. However, asymptomatic or latent CMV coinfection has been associated with CD8 T-cell elevation and immune activation in PLWH, leading to a lower CD4/CD8 ratio [14–16]. Moreover, elevation of anti-CMV IgG levels has been shown to be associated with neurocognitive dysfunction and cardiovascular disease in ART-treated PLWH [17–19]. These findings suggest that antiviral drugs may help alleviate chronic immune activation and inflammation in PLWH also by reducing CMV burden. Indeed, in 2011, Hunt et al found that daily administration of the antiviral valganciclovir for 8 weeks led to reduced circulating CMV DNA and immune activation in a group of 30 ART-treated PLWH with asymptomatic CMV coinfection [20].
CMV replication in the gut and subsequent epithelial gut damage have been shown to drive inflammation. In 2017, Maidji et al reported that CMV infection disrupted tight junctions and reduced epithelial integrity in the gut of 12 PLWH [5]. As Canada has been recently reported to have the second-lowest CMV seroprevalence among the general population in the world and a relatively low frequency in PLWH, our Canadian cohorts offer a unique opportunity to study the contribution of CMV coinfection to microbial translocation [21, 22]. Thus, we sought to investigate whether CMV serostatus and elevated anti-CMV IgG levels were independently associated with increased microbial translocation in well-defined groups of ART-naive and ART-treated PLWH.
METHODS
Description of Participants
A cross-sectional study was conducted on 150 adult PLWH from the Chronic Viral Illness Service at the McGill University Health Centre, the Montreal Primary HIV Infection Study, and the Canadian HIV and Aging Cohort Study as previously reported [23, 24]. A total of 26 HIV-uninfected controls were recruited from the Montreal Primary HIV Infection Study and the Canadian HIV and Aging Cohort Study who were either relatives or partners of PLWH. Participants were excluded if they presented with any symptomatic infection (including sexually transmitted infections such as gonorrhea, syphilis, and chlamydia) as well as hepatitis B or C coinfection. Antiretroviral drug classes, sociodemographic characteristics (including age, sex, race, education, and annual income), and behavioral characteristics (including sexual practices and recreational drug use) were recorded at study enrollment.
Laboratory Measurements
Fasting blood samples were collected from participants to isolate peripheral blood mononuclear cells and plasma, which were then stored in liquid nitrogen and at ˗80oC, respectively. Plasma HIV-1 p24 antigen/antibody and a confirmatory Western blot were used to establish the diagnosis of HIV infection, as previously reported [24]. Quantification of HIV plasma viral load was done using the Abbott RealTime HIV-1 assay (Abbott Laboratories, Abbott Park, Illinois). CD4 and CD8 T-cell counts were measured using 4-color flow cytometry. CMV seropositivity was determined by the McGill University Health Centre laboratory using chemiluminescent microparticle immunoassay on the Architect analyzer (Abbott). Anti-CMV IgG levels were measured using the CMV IgG enzyme immunoassay test kit (GenWay Biotech, San Diego, California). Anti-EBV IgG levels were measured using the EBV IgG enzyme-linked immunosorbent assay (ELISA) (GenWay Biotech). Intestinal fatty acid binding protein (I-FABP), a marker of epithelial gut damage in PLWH [25], was quantified using an ELISA (Hycult Biotech, Uden, Netherlands). Soluble CD14 (sCD14) was measured by immunoassay (Quantikine, R&D Systems, Minneapolis, Minnesota). Lipopolysaccharide (LPS) was quantified using the human LPS ELISA kit (Cusabio, Wuhan, China). The (1→3)-β-d-glucan (BDG) levels were measured by the Fungitell Limulus Amebocyte Lysate assay (Associates of Cape Cod, Inc, East Falmouth, Massachusetts). CXCL13, a marker of immune activation, was measured using the Human CXCL13/BLC/BCA-1 Quantikine ELISA kit (R&D Systems) [26]. Total IgG, immunoglobulin M (IgM), and immunoglobulin A (IgA) were measured by the McGill University Health Centre using the Olympus AU5800 (Beckman Coulter). Subclasses of IgG (IgG1–4) were measured using ELISAs from eBioSciences (Saint Laurent, Quebec, Canada). Interleukin (IL) 1β, tumor necrosis factor alpha (TNF-α), IL-6, and IL-8 were measured using the Meso Scale Discovery U-Plex Pro-Inflammatory Combo 4 kit (Meso Scale Discovery, Rockville, Maryland). All measurements were done in duplicate.
Statistical Analyses
Statistical analyses were conducted using SPSS 24.0 (IBM SPSS, Chicago, Illinois) and GraphPad Prism 6.0 (GraphPad, La Jolla, California). Comparisons were conducted using nonparametric Mann-Whitney U test and Kruskal-Wallis test with Dunn post hoc test. Spearman rank correlation test was conducted to assess the associations between quantitative variables. An α-level of 5% was used for statistical significance. As hypothesis testing of multiple parameters is prone to false positives, we sought to correct for this false discovery rate <0.05 using the Benjamini-Hochberg method (reported as Q value). Multivariate linear regression analysis was conducted to determine the independent association of anti-CMV IgG level with markers of epithelial gut damage, microbial translocation, and inflammation adjusting for age, sex, ethnicity, education, sexual practices, smoking and alcohol consumption, CD4 and CD8 T-cell counts, CD4/CD8 ratio, total IgG, total IgM, total IgA, subclasses IgG1–4, and anti-CMV IgG levels.
Ethical Considerations
Ethical approval was obtained from the McGill University Health Centre ethics board, as well as all research ethics boards of participating and recruiting centers. All study participants provided written consent. The study was conducted in accordance with the Declaration of Helsinki.
RESULTS
Participant Characteristics
Participants were grouped according to HIV seropositivity, ART usage, and CMV seropositivity. Sociodemographic and behavioral characteristics were similar among participants apart from participants of Hispanic origin among ART-naive PLWH (Table 1). The majority of ART-treated participants were on regimens of nucleoside reverse transcriptase inhibitors in combination with nonnucleoside reverse transcriptase inhibitors, protease inhibitors, or integrase strand transfer inhibitors (Supplementary Table 1). PLWH had similar CD4 T-cell count regardless of CMV serostatus in both the ART-naive and ART-treated groups. In contrast, elevated CD8 T-cell count and decreased CD4/CD8 ratio were associated with CMV serostatus among ART-naive and ART-treated PLWH. HIV viral load did not significantly differ with CMV serostatus among ART-naive PLWH. Total immunoglobulin IgG, IgM, IgA, and IgG1–4 did not differ with CMV serostatus within the same group (Supplementary Table 2). Median anti-EBV IgG levels were similar among CMV-seropositive and -seronegative participants (Table 1).
Table 1.
Sociodemographic, Behavioral, and Laboratory Measurements of Study Participants (N = 176)
| Living with HIV (n = 150 [85%]) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ART-Naive (n = 79 [53%]) | ART-Treated (n = 71 [47%]) | HIV-Uninfected (n = 26 [15%]) | |||||||
| Characteristics | CMV– (n = 13 [16%]) | CMV+ (n = 66 [84%]) | P Value | CMV– (n = 18 [25%]) | CMV+ (n = 53 [75%]) | P Value | CMV– (n = 13 [50%]) | CMV+ (n = 13 [50%]) | P Value |
| Sociodemographic characteristics | |||||||||
| Age, y | 36 (31–47) | 37 (32–41) | .89 | 43 (40–51) | 46 (40–49) | .79 | 52 (47–57) | 50 (45–58) | .87 |
| Male sex, No. (%) | 12 (92) | 63 (96) | .78 | 17 (97) | 48 (90) | .83 | 11 (85) | 10 (77) | .75 |
| Race/ethnicity, No. (%) | |||||||||
| White | 8 (62) | 47 (72) | .14 | 13 (73) | 37 (69) | .07 | 9 (69) | 9 (69) | .99 |
| African Canadian | 3 (23) | 17 (25) | .81 | 4 (22) | 14 (27) | .08 | 3 (23) | 3 (23) | .99 |
| Hispanic | 2 (15) | 2 (3) | .03 | 1 (5) | 2 (4) | .21 | 1 (8) | 1 (8) | .99 |
| Education, No. (%) | |||||||||
| Less than high school | 3 (23) | 17 (26) | .23 | 5 (25) | 15 (29) | .72 | 3 (23) | 2 (15) | .93 |
| High school and above | 7 (54) | 30 (45) | .08 | 7 (40) | 22 (41) | .99 | 6 (47) | 7 (54) | .96 |
| Annual income <$30 000 | 3 (23) | 19 (29) | .14 | 6 (35) | 16 (30) | .85 | 4 (30) | 4 (30) | .89 |
| Behavioral characteristics | |||||||||
| Sexual practices, No. (%) | |||||||||
| MSM | 10 (78) | 50 (76) | .82 | 12 (70) | 39 (73) | .38 | 9 (69) | 8 (62) | .41 |
| Heterosexual | 2 (17) | 13 (20) | .76 | 5 (25) | 12 (23) | .77 | 4 (31) | 3 (23) | .65 |
| Bisexual | 1 (5) | 3 (4) | .98 | 1 (5) | 2 (4) | .96 | 1 (8) | 2 (15) | .97 |
| Smoking and alcohol consumption, No. (%) | |||||||||
| Ex-smoker | … | … | 7 (39) | 19 (35) | .45 | 5 (42) | 5 (36) | .32 | |
| Current smoker | … | … | 4 (21) | 13 (25) | .78 | 3 (22) | 3 (23) | .71 | |
| Current alcohol use | … | … | 12 (68) | 35 (65) | .83 | 8 (62) | 8 (62) | .81 | |
| Laboratory measurements | |||||||||
| CD4+ T-cell count, cells/μL | 397 (253–510) | 406 (298–526) | .23 | 603 (407–700) | 588 (492–699) | .51 | 711 (598–924) | 779 (614–1005) | .52 |
| CD8+ T-cell count, cells/μL | 769 (620–1035) | 877 (703–1102) | .01 | 653 (502–783) | 760 (593–826) | .03 | 376 (291–513) | 379 (246–612) | .87 |
| CD4/CD8 T-cell ratio | 0.52 (0.45–0.89) | 0.46 (0.38–0.54) | .05 | 0.92 (0.87–1.10) | 0.77 (0.53–0.94) | .01 | 2.14 (2.09–2.27) | 2.39 (1.82–2.61) | .49 |
| HIV type 1 VL, log10 copies/mL | 4.57 | 4.54 | .97 | <1.6 | <1.6 | NA | NA | NA | NA |
| Duration of HIV infection, y | 0.28 | 0.26 | .82 | 14.2 | 15.7 | .79 | NA | NA | NA |
| Time to initiate ART, y | NA | NA | NA | 2.9 | 2.4 | .91 | NA | NA | NA |
| Duration on ART, y | NA | NA | NA | 12.3 | 13.4 | .84 | NA | NA | NA |
| Anti-CMV IgG, IU/mL | NA | 23.1 (8.7–33.4) | NA | NA | 24.3 (15.1–36.8) | NA | NA | 19.7 (9.6–31.4) | NA |
| Anti-EBV IgG, IU/mL | 16.0 (11.6–19.3) | 16.5 (12.8–19.2) | 0.97 | 15.2 (11.2–17.3) | 15.3 (11.9–18.6) | .96 | 13.2 (4.8–18.3) | 13.9 (5.6–20.8) | .92 |
Data are shown as median (interquartile range) unless otherwise stated. Currency is in Canadian dollars. Values in bold indicate P-value less than or equal to .05.
Abbreviations: –, seronegative; +, seropositive; …, not available; ART, antiretroviral therapy; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HIV, human immunodeficiency virus; IgG, immunoglobulin G; MSM, men who have sex with men; NA, not applicable; VL, viral load.
CMV Serostatus Was Associated With Increased Gut Damage and Microbial Translocation
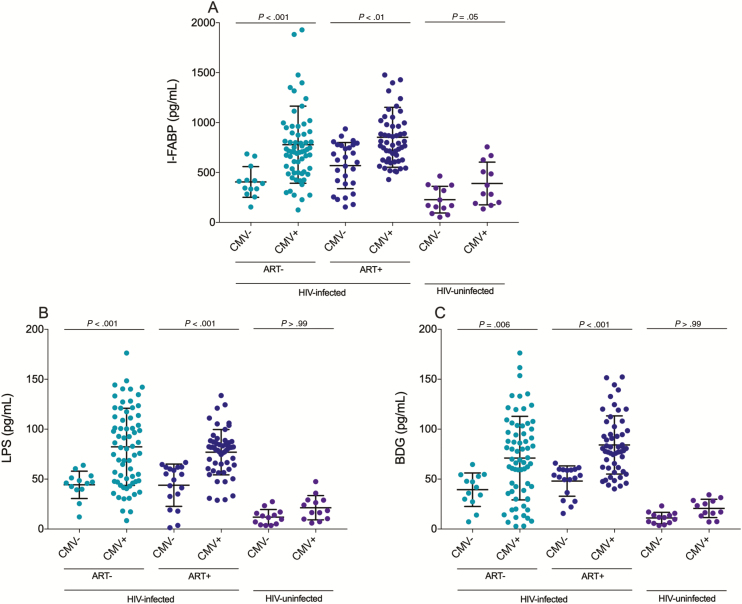
Circulating I-FABP, a marker of epithelial gut damage in HIV infection [27], was elevated in CMV-seropositive ART-naive PLWH (P < .001), ART-treated PLWH (P < .01), and HIV-uninfected participants (P = .05) compared with their CMV-seronegative counterparts (Figure 1A). Plasma levels of LPS were elevated in CMV-seropositive ART-naive (P < .001) and ART-treated PLWH (P < .001) only. Circulating LPS was not elevated in CMV-seropositive HIV-uninfected individuals (P > .99) (Figure 1B). Circulating fungal polysaccharide BDG, a marker of fungal translocation, was elevated in CMV-seropositive ART-naive (P = .006) and ART-treated PLWH (P < .001), but not in HIV-uninfected participants (P > .99) compared with their CMV-seronegative counterparts (Figure 1C). CMV-seropositive ART-naive PLWH had higher plasma levels of sCD14 than their CMV-seronegative counterparts (P = .002, data not shown). As previously reported by Freeman et al, plasma levels of sCD14 were similar among CMV-seropositive and -seronegative ART-treated PLWH (P = .91) and HIV-uninfected participants (P = .53) (data not shown) [14].
Figure 1.
Plasma levels of markers of epithelial gut damage and microbial translocation are elevated in antiretroviral therapy (ART)–naive and ART-treated cytomegalovirus (CMV)–seropositive people living with human immunodeficiency virus (PLWH). A, CMV-seropositive ART-naive and ART-treated PLWH as well as participants without human immunodeficiency virus (HIV) infection have higher plasma levels of intestinal fatty acid binding protein (I-FABP) compared with their CMV-uninfected counterparts. B, CMV-seropositive ART-naive and ART-treated PLWH have higher plasma levels of lipopolysaccharide (LPS) compared with their CMV-seronegative counterparts. C, CMV-seropositive ART-naive and ART-treated PLWH have higher plasma levels of (1→3)-β-d-glucan (BDG) compared with their CMV-seronegative counterparts. Horizontal lines represent first quartile, median, and third quartile, respectively. P values show Kruskal-Wallis tests with Dunn post hoc test between different groups. Light blue: ART-naive PLWH; dark blue: ART-treated PLWH; purple: participants without HIV infection.
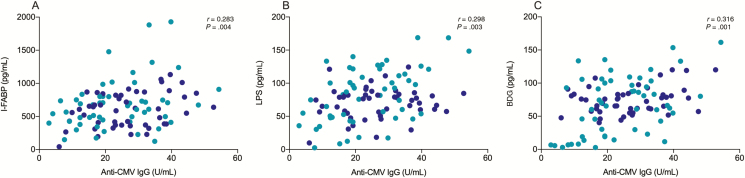
Anti-CMV IgG Levels Were Associated With Plasma Level Markers of Epithelial Gut Damage and Microbial Translocation
Elevated anti-CMV IgG levels have been previously associated with increased development of non-AIDS comorbidities in PLWH [18, 19]. We investigated whether anti-CMV IgG levels were associated with degree of epithelial gut damage and microbial translocation. Anti-CMV IgG levels were associated with the marker of epithelial gut damage I-FABP (r = 0.283; P = .004) as well as the markers of microbial translocation LPS (r = 0.298; P = .003) and BDG (r = 0.316; P = .001) among CMV-seropositive PLWH (Figure 2A–C). Such associations remained significant after adjusting for the rate of type I errors caused by hypothesis testing with multiple comparisons using false discovery rate <0.05 (Table 2). Importantly, total IgG, IgM, IgA, IgG1–4, and anti-EBV IgG levels were not associated with such markers. Among participants without HIV infection, anti-CMV IgG levels were also associated with plasma levels of I-FABP (r = 0.549; P = .04), LPS (r = 0.783; P = .004), and BDG (r = 0.633; P = .01) (Supplementary Figure 1). Again, such markers were not associated with total IgG, IgM, IgA, or IgG1–4, nor anti-EBV IgG among participants without HIV infection (Table 2). Multivariate analyses confirmed that the associations between anti-CMV IgG levels and markers of epithelial gut damage along with microbial translocation were independent of sociodemographic, behavioral, and laboratory characteristics (Table 1). Plasma levels of I-FABP, LPS, and BDG were similar among all ART-treated PLWH regardless of ART drug class (Supplementary Table 1).
Figure 2.
Anti-cytomegalovirus (CMV) immunoglobulin G (IgG) levels are associated with plasma levels of intestinal fatty acid binding protein (I-FABP), a marker of gut damage, and the microbial products lipopolysaccharide (LPS) and (1→3)-β-d-glucan (BDG) among CMV-coinfected people living with human immunodeficiency virus (PLWH). A, Anti-CMV IgG levels are associated with plasma levels of I-FABP (n= 119). B, Plasma levels of anti-CMV IgG are associated with plasma levels of LPS (n= 119). C, Anti-CMV IgG levels are associated with plasma levels of BDG (n= 119). P values show nonparametric Spearman correlations. Light blue: ART-naive PLWH; dark blue: ART-treated PLWH.
Table 2.
Correlations and False Discovery Rate Adjusted P value (Q Value) of Plasma-level Markers of Epithelial Gut Damage, Microbial Translocation, and Inflammation With Anti-Cytomegalovirus (CMV) and Anti–Epstein-Barr Virus Immunoglobulin G Among CMV-Infected Participants (n = 132)
| Living with HIV (n = 119 [90%]) | ||||||
|---|---|---|---|---|---|---|
| ART-Naive (n = 66 [55%]) | ART-Treated (n = 53 [45%]) | HIV-Uninfected (n = 13 [10%]) | ||||
| Plasma Markers | Anti-CMV IgG | Anti-EBV IgG | Anti-CMV IgG | Anti-EBV IgG | Anti-CMV IgG | Anti-EBV IgG |
| Gut damage | ||||||
| I-FABP | r = 0.299 | r = 0.032 | r = 0.296 | r = 0.015 | r = 0.549 | r = ˗0.02 |
| P = .03 | P = .47 | P = .04 | P = .35 | P = .04 | P = .96 | |
| Q = .04 | Q = .51 | Q = .05 | Q = .51 | Q = .05 | Q = .87 | |
| Microbial translocation | ||||||
| LPS | r = 0.455 | r = 0.002 | r = 0.201 | r = ˗0.001 | r = 0.783 | r = 0.003 |
| P < .001 | P = .21 | P = .01 | P = .22 | P = .004 | P = .89 | |
| Q = .002 | Q = .32 | Q = .05 | Q = .39 | Q = .007 | Q = .85 | |
| BDG | r = 0.403 | r = 0.033 | r = 0.298 | r = 0.051 | r = 0.633 | r = ˗0.014 |
| P < .001 | P = .56 | P = .02 | P = .42 | P = .01 | P = .97 | |
| Q = .001 | Q = .58 | Q = .03 | Q = .68 | Q = .03 | Q = .99 | |
| Inflammation | ||||||
| CXCL13 | r = 0.254 | r = 0.318 | r = 0.623 | r = 0.271 | r = 0.704 | r = 0.388 |
| P = .05 | P = .01 | P < .001 | P = .06 | P = .01 | P = .08 | |
| Q = .09 | Q = .04 | Q < .001 | Q = .10 | Q = .04 | Q = .13 | |
| IL-1β | r = 0.351 | r = 0.219 | r = 0.209 | r = 0.001 | r = 0.469 | r = 0.245 |
| P = .01 | P = .13 | P = .04 | P > .99 | P = .128 | P = .44 | |
| Q = .04 | Q = .22 | Q = .09 | Q > .99 | Q = .178 | Q = .42 | |
| IL-6 | r = 0.173 | r = 0.201 | r = 0.401 | r = 0.193 | r = 0.419 | r = 0.041 |
| P = .221 | P = .09 | P= .004 | P = .10 | P = .177 | P = .91 | |
| Q = .128 | Q = .14 | Q= .01 | Q = .21 | Q = .201 | Q > .99 | |
| IL-8 | r= 0.430 | r = 0.188 | r= 0.333 | r = 0.102 | r = 0.259 | r = ˗0.511 |
| P < .001 | P = .28 | P= .02 | P = .34 | P = .413 | P = .09 | |
| Q= .003 | Q = .31 | Q= .04 | Q = .44 | Q = .397 | Q = .07 | |
| TNF-α | r= 0.419 | r = 0.221 | r= 0.274 | r = 0.154 | r = 0.574 | r = 0.080 |
| P= .002 | P = .05 | P = .01 | P = .21 | P = .05 | P = .81 | |
| Q= .02 | Q = .08 | Q= .05 | Q = .38 | Q = .09 | Q = .95 | |
Data in bold indicate significant association after adjustment for false discovery rate (< 0.05).
Abbreviations: ART, antiretroviral therapy; BDG, (1→3)-β-d-glucan; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HIV, human immunodeficiency virus; I-FABP, intestinal fatty acid binding protein; IgG, immunoglobulin G; IL, interleukin; LPS, lipopolysaccharide; TNF, tumor necrosis factor.
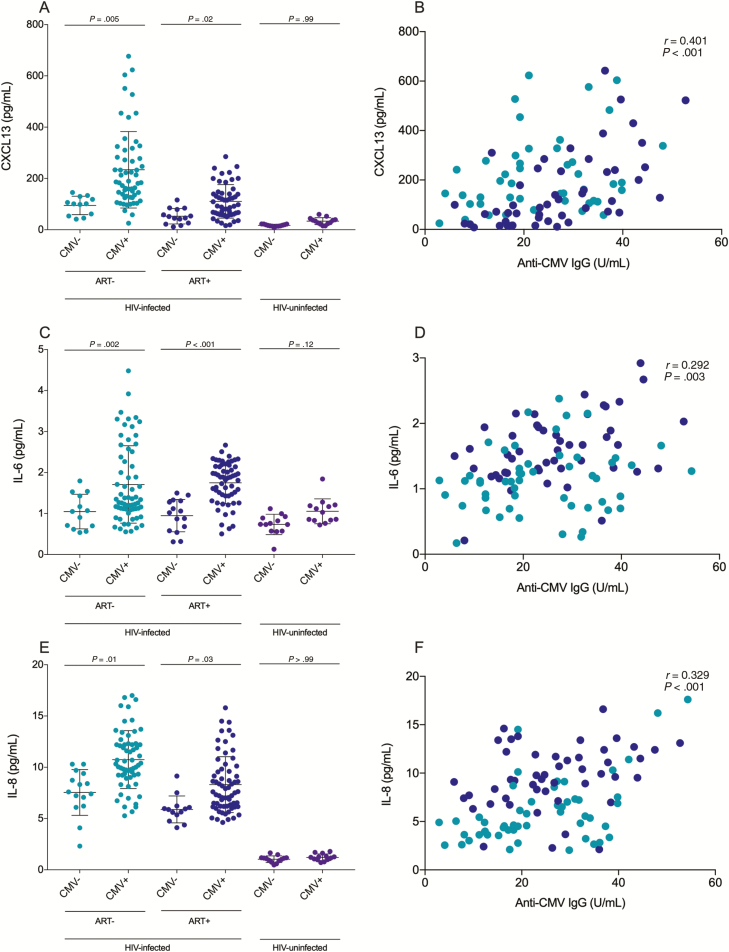
CMV Seropositivity Was Associated With Increased Systemic Inflammation
CMV-seropositive ART-naive and ART-treated PLWH had higher plasma levels of CXCL13, a marker of immune activation (P = .005 and P = .02, respectively; Figure 3A), IL-6 (P = .02 and P < .001; Figure 3C), and IL-8 (P = .01 and P = .03; Figure 3E). However, plasma levels of IL-1β and TNF-α were not associated with CMV seropositivity (Supplementary Figure 2). Anti-CMV IgG levels were associated with plasma levels of CXCL13 (r = 0.401; P < .001; Figure 3B) [28], IL-6 (r = 0.292; P = .003; Figure 3D), IL-8 (r = 0.329; P < .001; Figure 3E), IL-1β (r = 0.281; P = .005), and TNF-α (r = 0.217; P = .03; Supplementary Figure 2) in CMV-seropositive PLWH. Among participants without HIV infection, anti-CMV IgG level was associated with plasma levels of CXCL13 (r = 0.704; P = .01) and TNF-α (r = 0.574; P = .05) but not with plasma levels of IL-1β (r = 0.469; P = .128), IL-6 (r = 0.419; P = .177), or IL-8 (r = 0.259; P = .413) (Supplementary Figure 1). Anti-EBV IgG levels were associated with plasma levels of CXCL13 in ART-naive PLWH (r = 0.318; P = .01) but not in ART-treated PLWH (r = 0.271; P = .06) nor participants without HIV infection (r = 0.388; P = .08). Total IgG, IgM, IgA, IgG1–4, and anti-EBV IgG were not associated with plasma levels of IL-1β, IL-6, IL-8, or TNF-α (Table 2). Multivariate analyses confirmed that anti-CMV IgG levels were associated with plasma levels of CXCL13, IL-1β, IL-6, IL-8, and TNF-α independent of sociodemographic and behavioral characteristics as well as laboratory measurements (Table 1). Plasma levels of CXCL13, IL-1β, IL-6, IL-8, and TNF-α were similar among all ART-treated PLWH regardless of ART drug class (Supplementary Table 1).
Figure 3.
Plasma levels of marker of inflammation CXCL13 and the proinflammatory cytokines interleukin (IL) 6 and IL-8 are elevated in cytomegalovirus (CMV)–coinfected people living with human immunodeficiency virus (HIV), ie, PLWH. A, CMV-seropositive antiretroviral therapy (ART)–naive and ART-treated PLWH have higher plasma levels of CXCL13 compared with their CMV-seronegative counterparts. B, Plasma levels of anti-CMV immunoglobulin G (IgG) are associated with plasma levels of CXCL13 in CMV-infected PLWH (n= 119). C, CMV-seropositive ART-naive and ART-treated PLWH have higher plasma levels of IL-6 compared with their CMV-seronegative counterparts. D, Plasma levels of anti-CMV IgG are associated with plasma levels of IL-6 in CMV-infected PLWH (n= 119). E, CMV-seropositive ART-naive and ART-treated PLWH have higher plasma levels of IL-8 compared with their CMV-seronegative counterparts. F, Plasma levels of anti-CMV IgG are associated with plasma levels of IL-8 in CMV-infected PLWH (n= 119). Horizontal lines represent first quartile, median, and third quartile, respectively. P values show Kruskal-Wallis tests with Dunn post hoc test between different groups. Associations with anti-CMV IgG levels show nonparametric Spearman correlations. Light blue: ART-naive PLWH; dark blue: ART-treated PLWH; purple: people without HIV infection.
DISCUSSION
In this cross-sectional study, we investigated the contribution of CMV seropositivity on microbial translocation and inflammation in ART-naive and ART-treated PLWH. In line with previous studies, we confirmed that CMV seropositivity was associated with higher CD8 T-cell counts and lower CD4/CD8 ratio in PLWH [14, 29, 30]. Moreover, we expanded upon such observations by showing that CMV seropositivity and anti-CMV IgG levels were associated with higher plasma levels of markers of gut damage (I-FABP), bacterial (LPS) and fungal (BDG) translocation, and proinflammatory cytokines in both ART-naive and ART-treated PLWH as well as uninfected controls.
CMV seropositivity was associated with increased epithelial gut damage and microbial translocation markers independent of CD8 T-cell count and CD4/CD8 ratio. Furthermore, markers of epithelial gut damage, bacterial translocation, and fungal translocation were associated with anti-CMV IgG levels but not anti-EBV IgG levels nor total IgG, IgM, IgA, and IgG1–4. These findings further support the observation that increased epithelial gut damage and microbial translocation are linked in part to CMV seropositivity and not influenced by systemic inflammation nor global immunoglobulin levels. Moreover, epithelial gut damage and microbial translocation markers were positively associated with anti-CMV IgG levels. In elderly populations, higher levels of anti-CMV IgG were associated with CMV reactivation and aging outcomes [31]. Conversely, anti-EBV IgG level was not associated with CD8 T-cell elevation, epithelial gut damage, microbial translocation, nor inflammatory markers apart from plasma levels of CXCL13 in ART-naive PLWH. We and others have recently showed that CXCL13, a marker of lymph node germinal center activity, can also be considered as a novel marker of HIV disease progression, inflammation, and T-cell activation in PLWH [26]. This observation is in line with some EBV-related non-Hodgkin lymphomas where circulating CXCL13 level is used as prognostic score for survival outcome [32].
In line with our findings, Maidji et al have recently observed that the gastrointestinal tract is one of the largest sites of CMV replication [5]. Moreover, primary human intestinal cells and humanized mouse models of the gut showed that CMV infection, independent of HIV, disrupted tight junctions of polarized intestinal cells and enhanced transepithelial permeability contributing to microbial translocation [5]. Additionally, CMV infection has been shown to induce IL-6 and IL-8 production in human enterocytes in vitro and in vivo, respectively [5, 33]. Likewise, we report that CMV seropositivity and anti-CMV IgG levels were associated with higher plasma levels of IL-6 and IL-8 among both ART-naive and ART-treated PLWH. We observed a trend between CMV seropositivity and elevated plasma levels of IL-1β and TNF-α; however, this tendency was not statistically significant. This is in line with previous findings from Maidji et al that show an induction of IL-6 production but not IL-1β nor TNF-α in vitro in human colon epithelial cells following CMV infection [5]. Thus, with our findings, there is converging in vitro and ex vivo evidence that CMV infection is a contributor to epithelial gut damage and inflammation.
Despite the significant success of ART, chronic immune activation and resultant risk of non-AIDS comorbidities persist [34, 35]. Our findings, combined with those of previous studies, suggest that CMV coinfection may exacerbate epithelial gut damage in PLWH, contributing to increased microbial translocation and inflammation even after long-term ART [5]. As such, targeting CMV may help alleviate epithelial gut damage in ART-treated PLWH and reduce the incidence of non-AIDS comorbidities.
We acknowledge certain limitations to our study. While we assessed CMV seropositivity and used anti-EBV IgG levels as a Herpesviridae family control, we did not investigate the contribution of other coinfections common among PLWH such as other herpesviruses (types 1, 2, and 8), hepatitis B, and hepatitis C. Furthermore, although anti-CMV IgG levels are not a marker of CMV replication, we compared people who are seropositive for CMV with people who are seronegative [36, 37]. The differences between these 2 groups as well as the associations with anti-CMV IgG but not anti-EBV IgG levels suggest that CMV coinfection is playing an active role in persistent epithelial gut damage in ART-treated PLWH. Further evaluation of CMV replication in blood and tissues with the use of quantitative polymerase chain reaction is needed to appraise the contribution of transient CMV replication in tissue on the elevation of plasma levels of anti-CMV IgG.
Overall, we reported for the first time that CMV-seropositive ART-naive and ART-treated PLWH have increased microbial translocation and inflammation compared to those without CMV seropositivity. Anti-CMV IgG levels were also correlated with markers of epithelial gut damage, microbial translocation, and inflammation independent of anti-EBV IgG and other nonspecific immunoglobulins. Furthermore, such observations were independent of sociodemographic and behavioral characteristics. CMV infection may explain the persistence of epithelial gut damage, microbial translocation, and immune activation in PLWH on long-term ART and in uninfected controls. Future studies should assess whether anti-CMV therapy can alleviate epithelial gut damage, microbial translocation, and chronic inflammation in ART-treated PLWH [38].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. R. R. performed the experiments, analyzed the data, wrote the first draft, and revised the final draft of the manuscript. S. I., J. L., B. F., J. O., V. M., Y. Z., and M. F. contributed to the experiments, data analysis, and critical review of the manuscript. B. L., C. C., C. C. L., M. D., C. T., P. A., and J.-P. R. all contributed to the recruitment and follow-up of study participants and critically reviewed the manuscript. G. B. provided insightful advice in the preparation of the first draft and critically reviewed the final draft of the manuscript.
Acknowledgments. The authors are highly grateful to the study participants for their contributions; Angie Massicotte, Josée Girouard, and Jacquie Sas for study coordination and assistance; and Mario Legault and Stéphanie Matte for coordinating the Montreal Primary HIV Infection Study, the Canadian HIV and Aging Cohort Study, and the Canadian Cohort of HIV-Infected Slow Progressors. The authors also thank the following physicians for recruitment and follow-up of study participants, all of whom were in Montreal, Canada: R. Thomas, C. Milne, S. Lavoie, J. Friedman, M. Duchastel, F. Villielm, F. Asselin, M. Boissonnault, P. J. Maziade, S. Lavoie, M. Milne, N. Z. Miaki, and M. E. Thériault (Clinique médicale l’Actuel); B. Lessard, M. A. Charron, S. Dufresne, M. E. Turgeon, S. Vézina, E. Huchet, J. P. Kerba, M. Poliquin, S. Poulin, P. Rochette, P. Junod, D. Longpré, R. Pilarski, E. Sasseville, L. Charest, A. Hamel, A. Cloutier-Blais, S. Massoud, F. Chano, P. Coté, D. Dufrenes, and B. Trottier (Clinique médicale urbaine du Quartier Latin); L. Labrecque, C. Fortin, V. Hal-Gagne, M. Munoz, B. Deligne, V. Martel-Laferrière, and M. E. Goyer (Centre Hospitalier de l’Université de Montréal); M. Teltscher, A. de Pokomandy, J. Cox, J. Szabo, M. Klein, and L. P. Haraoui (McGill University Health Centre Chronic Viral Illness Service).
Financial support. This study was financed by the Fonds de la Recherche Québec-Santé (FRQ-S): Réseau SIDA/Maladies infectieuses and Thérapie cellulaire; Réseau de bioimagerie du Québec (FRQ-S); the Canadian Institutes of Health Research (CIHR; grant numbers MOP 103230 and PTJ 166049); the Vaccines and Immunotherapies Core of the CIHR Canadian HIV Trials Network (grant number CTN 257); Département de radiologie, radio-oncologie et médecine nucléaire, University of Montreal; the National Institutes of Health; the Canadian Foundation for AIDS Research (grant number 02-512); and the CIHR-funded Canadian HIV Cure Enterprise (team grant number HB2-164064). R. R. is an undergraduate student supported by the H. Grenville Smith Studentship. S. I. is a postdoctoral fellow supported by the William Turner research fellowship and Normand Fortier foundation. J. P. R. is the holder of the Louis Lowenstein Chair in Hematology and Oncology, McGill University and William Turner award holder from the McGill University Health Centre. M. D. is supported by a salary award from the FRQ-S.
Potential conflicts of interest. Y. Z. and M. F. are employees of Associates of Cape Cod, Inc, the manufacturers of Fungitell, the (1→3)-β-d-glucan in vitro diagnostic kit. T. C. reports grants from Merck, Gilead, and ViiV, and personal fees from Merck, outside the submitted work. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Klenerman P, Oxenius A. T cell responses to cytomegalovirus. Nat Rev Immunol 2016; 16:367–77. [DOI] [PubMed] [Google Scholar]
- 2. Fakhreddine AY, Frenette CT, Konijeti GG. A practical review of cytomegalovirus in gastroenterology and hepatology. Gastroenterol Res Pract 2019; 2019:6156581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bajwa M, Vita S, Vescovini R, et al. . CMV-specific T-cell responses at older ages: broad responses with a large central memory component may be key to long-term survival. J Infect Dis 2017; 215:1212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collins-McMillen D, Buehler J, Peppenelli M, Goodrum F. Molecular determinants and the regulation of human cytomegalovirus latency and reactivation. Viruses 2018; 10. doi:10.3390/v10080444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maidji E, Somsouk M, Rivera JM, Hunt PW, Stoddart CA. Replication of CMV in the gut of HIV-infected individuals and epithelial barrier dysfunction. PLoS Pathog 2017; 13:e1006202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paiardini M, Müller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev 2013; 254:78–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hensley-McBain T, Berard AR, Manuzak JA, et al. . Intestinal damage precedes mucosal immune dysfunction in SIV infection. Mucosal Immunol 2018; 11:1429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoenigl M, de Oliveira MF, Pérez-Santiago J, et al. . (1→3)-β-D-glucan levels correlate with neurocognitive functioning in HIV-infected persons on suppressive antiretroviral therapy: a cohort study. Medicine 2016; 95:e3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brenchley JM, Price DA, Schacker TW, et al. . Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–71. [DOI] [PubMed] [Google Scholar]
- 10. Hoenigl M, Moser C, Funderburg N, et al. . Soluble urokinase plasminogen activator receptor (suPAR) is predictive of non-AIDS events during antiretroviral therapy-mediated viral suppression [manuscript published online ahead of print 12 November 2018]. Clin Infect Dis 2018. doi:10.1093/cid/ciy966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mehraj V, Ramendra R, Isnard S, et al. . Circulating (1-->3)-beta-D-glucan is associated with immune activation during HIV infection [manuscript published online ahead of print 16 March 2019]. Clin Infect Dis 2019. doi:10.1093/cid/ciz212. [Google Scholar]
- 12. Hunt PW, Sinclair E, Rodriguez B, et al. . Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014; 210:1228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gianella S, Letendre S. Cytomegalovirus and HIV: a dangerous pas de deux. J Infect Dis 2016; 214(Suppl 2):S67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Freeman ML, Mudd JC, Shive CL, et al. . CD8 T-cell expansion and Inflammation linked to CMV coinfection in ART-treated HIV infection. Clin Infect Dis 2016; 62:392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu W, Mehraj V, Vyboh K, Cao W, Li T, Routy JP. CD4:CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV-positive patients. J Int AIDS Soc 2015; 18:20052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poizot-Martin I, Allavena C, Duvivier C, et al. . Dat’AIDS Study Group CMV+ serostatus associates negatively with CD4:CD8 ratio normalization in controlled HIV-infected patients on cART. PLoS One 2016; 11:e0165774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parrinello CM, Sinclair E, Landay AL, et al. . Cytomegalovirus immunoglobulin G antibody is associated with subclinical carotid artery disease among HIV-infected women. J Infect Dis 2012; 205:1788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lichtner M, Cicconi P, Vita S, et al. . ICONA Foundation Study Cytomegalovirus coinfection is associated with an increased risk of severe non-AIDS-defining events in a large cohort of HIV-infected patients. J Infect Dis 2015; 211:178–86. [DOI] [PubMed] [Google Scholar]
- 19. Letendre S, Bharti A, Perez-Valero I, et al. . CNS HIV AntiRetroviral Therapy Effects Research (CHARTER) Group Higher anti-cytomegalovirus immunoglobulin G concentrations are associated with worse neurocognitive performance during suppressive antiretroviral therapy. Clin Infect Dis 2018; 67:770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hunt PW, Martin JN, Sinclair E, et al. . Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis 2011; 203:1474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barrett L, Stapleton SN, Fudge NJ, Grant MD. Immune resilience in HIV-infected individuals seronegative for cytomegalovirus. AIDS 2014; 28:2045–9. [DOI] [PubMed] [Google Scholar]
- 22. Zuhair M, Smit GSA, Wallis G, et al. . Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis. Rev Med Virol 2019; 29:e2034. [DOI] [PubMed] [Google Scholar]
- 23. Durand M, Chartrand-Lefebvre C, Baril JG, et al. . investigators of the Canadian HIV and Aging Cohort Study The Canadian HIV and aging cohort study—determinants of increased risk of cardio-vascular diseases in HIV-infected individuals: rationale and study protocol. BMC Infect Dis 2017; 17:611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mehraj V, Cox J, Lebouche B, et al. . Socio-economic status and time trends associated with early ART initiation following primary HIV infection in Montreal, Canada: 1996 to 2015. J Int AIDS Soc 2018; 21. doi:10.1002/jia2.25034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prendergast AJ, Chasekwa B, Rukobo S, et al. . Intestinal damage and inflammatory biomarkers in human immunodeficiency virus (HIV)-exposed and HIV-infected Zimbabwean infants. J Infect Dis 2017; 216:651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mehraj V, Ramendra R, Isnard S, et al. . CXCL13 as a biomarker of immune activation during early and chronic HIV infection. Front Immunol 2019; 10:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheru LT, Park EA, Saylor CF, et al. . I-FABP is higher in people with chronic HIV than elite controllers, related to sugar and fatty acid intake and inversely related to body fat in people with HIV. Open Forum Infect Dis 2018; 5:ofy288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kazanietz MG, Durando M, Cooke M. CXCL13 and its receptor CXCR5 in cancer: inflammation, immune response, and beyond. Front Endocrinol (Lausanne) 2019; 10:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cao W, Mehraj V, Kaufmann DE, Li T, Routy JP. Elevation and persistence of CD8 T-cells in HIV infection: the Achilles heel in the ART era. J Int AIDS Soc 2016; 19:20697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Routy JP, Mehraj V. Very early antiretroviral therapy permits CD8 T cells to keep HIV reservoirs at bay. Ann Transl Med 2017; 5:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parry HM, Zuo J, Frumento G, et al. . Cytomegalovirus viral load within blood increases markedly in healthy people over the age of 70 years. Immun Ageing 2016; 13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim DY, Joo YD, Lim SN, et al. . Adult Acute Lymphoblastic Leukemia Working Party of the Korean Society of Hematology Nilotinib combined with multiagent chemotherapy for newly diagnosed Philadelphia-positive acute lymphoblastic leukemia. Blood 2015; 126:746–56. [DOI] [PubMed] [Google Scholar]
- 33. Gianella S, Chaillon A, Mutlu EA, et al. . Effect of cytomegalovirus and Epstein-Barr virus replication on intestinal mucosal gene expression and microbiome composition of HIV-infected and uninfected individuals. AIDS 2017; 31:2059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hsu DC, Sereti I. Serious non-AIDS events: therapeutic targets of immune activation and chronic inflammation in HIV infection. Drugs 2016; 76:533–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ndumbi P, Gilbert L, Tsoukas CM. Comprehensive evaluation of the immune risk phenotype in successfully treated HIV-infected individuals. PLoS One 2015; 10:e0117039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tremblay MA, Rodrigue MA, Deschênes L, Boivin G, Longtin J. Cytomegalovirus quantification in plasma with Abbott RealTime CMV and Roche Cobas Amplicor CMV assays. J Virol Methods 2015; 225:1–3. [DOI] [PubMed] [Google Scholar]
- 37. Gianella S, Morris SR, Tatro E, et al. . Virologic correlates of anti-CMV IgG levels in HIV-1-infected men. J Infect Dis 2014; 209:452–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kotton CN, Kumar D, Caliendo AM, et al. . The Transplantation Society International CMV Consensus Group The Third International Consensus Guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2018; 102:900–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.