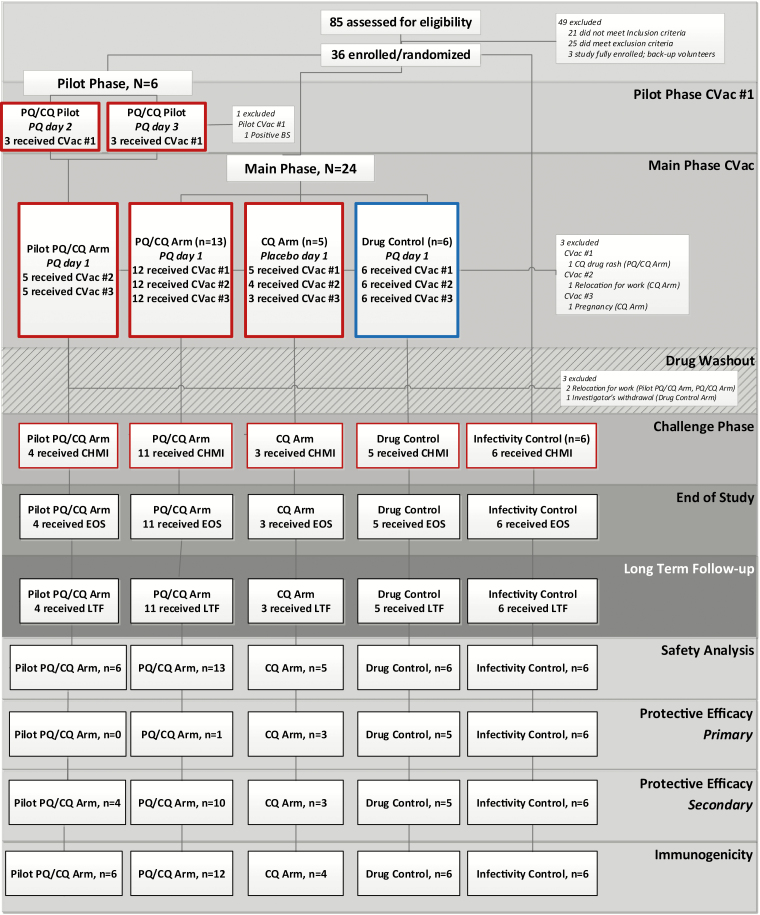
Figure 1.
Trial profile. The boxes outlined in red represent the receipt of Pf SPZ+ mosquito bites, and those outlined in blue represent the receipt of Pf SPZ- mosquito bites. All CVac subjects received CQ from 8 days prior to the first iteration of mosquito bites to 20 days following the third mosquito bite exposure. Approximately 5 weeks elapsed between the discontinuation of CQ and CHMI to allow drug concentrations to decrease to subtherapeutic concentrations. “Received CVac” was defined as undergoing Pf SPZ± mosquito bites and receiving PQ/placebo. The EOS was defined as the final post-CHMI study visit (study Day 182; 35 days post-CHMI). LTF visits at 3 and 6 months post-CHMI were optional. Abbreviations: -, noninfectious mosquito bites; +, infectious mosquito bites; CHMI, controlled human malaria infection; CQ, chloroquine; CVac, chemoprophylaxis vaccination with sporozoites; EOS, end of study; LTF, long-term follow-up; Pf, Plasmodium falciparum; PQ, primaquine; SPZ, sporozoites.