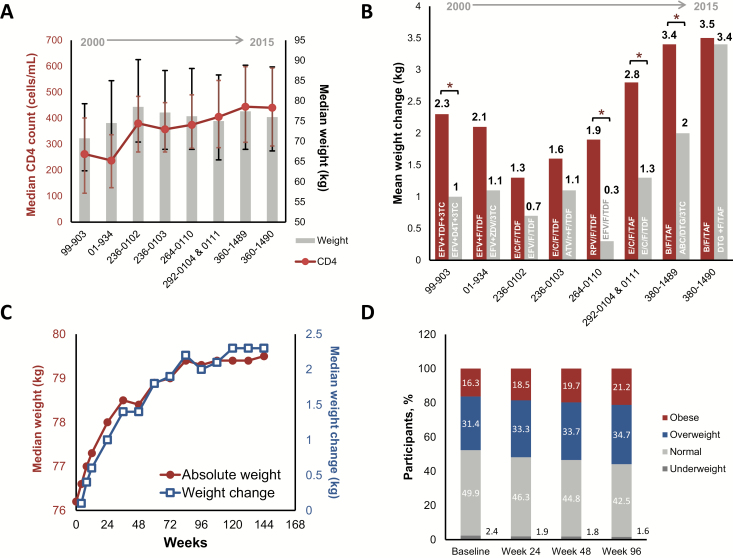
Figure 1.
Weight trends in participants initiating antiretroviral therapy. A, Baseline median CD4 cell count and median weight in the indicated clinical trials, which are ordered by date of trial initiation. Error bars represent the first through third quartiles. B, Mean weight change observed at the 48-week time point for the indicated trials, which are organized by date of initiation. Red bars are the investigational regimen, and gray bars are the comparator. *P < .05 by analysis of variance. C, Median weight (red) and median weight change (blue) over time in 8 pooled clinical trials. D, Body mass index category distributions over time in 8 pooled clinical trials. Abbreviations: 3TC, lamivudine; ABC, abacavir; ATV/r, ritonavir-boosted atazanavir; B, bictegravir; C, cobicistat; D4T, stavudine; DTG, dolutegravir; E, elvitegravir; EFV, efavirenz; F, emtricitabine; RPV, rilpivirine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine.