Abstract
Cefazolin and ertapenem combination therapy was used successfully to salvage 11 cases (6 endocarditis) of persistent methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia, including immediate clearance (≤24 hours) in 8 cases. While in vitro synergy was modest, cefazolin plus ertapenem exhibited synergistic action in a rat model of MSSA endocarditis. The combination of cefazolin and ertapenem provides potent in vivo activity against MSSA beyond what is predicted in vitro and warrants further clinical study in the treatment of refractory MSSA bacteremia and endocarditis.
Keywords: Staphylococcus aureus, bacteremia, endocarditis, cefazolin, ertapenem
Cefazolin and ertapenem combination therapy yielded profound clinical success in 11 severe methicillin-susceptible Staphylococcus aureus infections with high bacterial densities (6 endocarditis), as demonstrated by rapid bacteremia clearance. Enhanced efficacy was also observed in a rat endocarditis model.
Staphylococcus aureus is a major cause of bacteremia, causing significant morbidity and mortality in high-risk patients [1, 2]. Management of persistent methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia is grounded in surgical source control and early initiation of β-lactam therapy with classical antistaphylococcal agents (eg, oxacillin, nafcillin, flucloxacillin) or cefazolin [3]. While the latter is being increasingly utilized, infections with high MSSA inoculum, as in infective endocarditis, have been associated with clinical treatment failure in patients receiving cefazolin [4–7]. Although suboptimal therapy against MSSA may have catastrophic clinical consequences, currently there is no guidance on optimal treatment regimens for high-inoculum MSSA infections refractory to standard treatment regimens.
We previously described that adding ertapenem to cefazolin led to in vitro and in vivo synergy against an MSSA bloodstream isolate from a patient for whom this drug combination was used successfully in salvage therapy for persistent bacteremia without a surgical focus [8]. We have continued to experience high success with this salvage regimen for refractory MSSA bacteremia. Here we report our clinical experience using cefazolin plus ertapenem as salvage therapy for 11 cases with refractory MSSA bacteremia, and explore this combination therapy in vitro and in an established rat model of endocarditis.
METHODS
Patient Cases
Patients with persistent MSSA bacteremia were identified through treating physicians (authors G. S. and F. H.), and the following data were collected retrospectively: patient age, source of bacteremia, duration of bacteremia, hematology and chemistry laboratory tests, and prior antibiotics administered that failed to clear bacteremia. Of the 11 patients in the case report, 9 had daily blood cultures as part of their routine clinical management, which allowed for bacteremia duration assessment. For 2 of the patients, blood cultures were separated by 3 days from the time of the last positive to first negative, such that specific bacteremia duration could not be measured. These details are provided in the clinical summary (Table 1). Note that all patients treated with ertapenem plus cefazolin were included and no patients were excluded from the case report. Expedited approval for data collection was granted by the Sharp Healthcare Internal Review Board.
Table 1.
Use of Cefazolin Plus Ertapenem as Salvage Therapy for Persistent Methicillin-Susceptible Staphylococcus aureus Bacteremia
| Days of Bacteremia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case No. | Age/Sex | Prior Therapies (No. of Days) | Prior to ETP/CZ | After ETP/CZ | Source(s) | Comorbidities | IL-10, pg/mL | ICU | Comments | |
| 1 | 70s/F | PIP-TAZ (1) CZ (5) | 6 | 0 | Osteomyelitis Epidural abscess Lumbar spine | CHF/atrial fibrillation DM 2 COPD Obesity Eczema | 5 | No | Bedridden, morbid obesity, limited workup. This case was previously published [8]. | |
| 2 | 60s/F | PIP-TAZ (1) Nafcillin (3) | 4 | 0 | AV fistula Pneumonia | Renal transplant DM 2 | 9 | Yes | AV fistula placed 2 weeks prior. | |
| 3 | 20s/F | CZ (4 + 5)a | 16 9 on antibiotics | 0 | Tricuspid valve 2 × 2 cm vegetation | IVDU | Not done | No | Vegetation grew to 2.5 × 2.3 cm, but improved clinically to surgical candidacy (initially declined due to poor medical condition). Tricuspid valve repair 10 days after bacteremia clearance. | |
| 4 | 30s/M | Vancomycin + ceftriaxone (1), nafcillin (3) | 4 | 0 | Triscuspid valve, 2.3 × 3.3 cm and 1.2 × 1.4 cm vegetations | IVDU | 14 | Yes | AngioVac 3 days after bacteremia clearance. Partial success in vegetation debulking with 1.9 × 2 cm residual vegetation. Signed out against medical advice 25 days after bacteremia clearance. | |
| 5 | 50s/F | Vancomycin + ceftriaxone (1), nafcillin (4) | 5 | 0 | Psoas and L3–L1 epidural abscess | None | 7 | No | 48 hours into bacteremia developed neck pain, found to have C5–C7 cervical pachymeningitis/discitis; vulvar abscess. | |
| 6 | 60s/M | Vancomycin + PIP-TAZ (1), nafcillin (4) | 5 | 2 | Cervical paraspinous abscess and thigh abscess | DM 2 (HbA1C = 12 mg/dL) | 6 | No | Secondary suppurative pericarditis 2 weeks into treatment. | |
| 7 | 30s/M | Vancomycin + PIP-TAZ (1) Vancomycin + ceftriaxone (1), nafcillin (5) | 7 | ≤3b | Aortic valve Meningitis | IVDU | Not done | Yes | Cardioembolic shower with multiple septic emboli to brain. Cardiogenic shock with heart failure. Aortic valve replacement on hospital day 23. | |
| 8 | 30s/M | Vancomycin + PIP-TAZ (1) Vancomycin + ceftriaxone (1), nafcillin (3) Nafcillin + rifampin (3) | 8 | 0 | Tricuspid valve | IVDU Hepatitis C | Not done | No | Multiple septic pulmonary emboli. Empyema requiring decortication. | |
| 9 | 70s/F | Nafcillin (5) | 6 | 0 | Sacroiliac joint | ESRD DM 2 | 7 | No | ESRD patient admitted with uremia to initiate hemodialysis via AV fistula. Patient developed right hip/buttock pain and leukocytosis but remained afebrile and was discharged. She was called back to the hospital for positive blood cultures. | |
| 10 | 70s/F | Vancomycin + ceftriaxone (4), CZ (2) | 6 | ≤3b | Mitral valve Aortic valve | Hypertension Hyperlipidemia | Not done | Yes | Multiple brain emboli, intracranial hemorrhage, lumbar discitis, and osteomyelitis. | |
| 11 | 60s/M | Vancomycin + PIP-TAZ (2) CZ + gentamicin + rifampin (4) | 6 | 0 | Aortic valve 1.3 × 1 and 1 × 1 cm vegetation Aortic graft infection | Hypertension Atrial fibrillation Seizures Aortic aneurysm repair Cerebral aneurysm clipping Prior rectal cancer | Not done | No | Hospital day 8, patient underwent sternotomy, hemiarch and ascending aorta repair, aortic valve replacement (Edwards Magna), mediastinal debridement. Negative blood cultures 48 hours preoperatively on CZ plus ETP. Intraoperative tissue cultures + MSSA. Postoperative reinitiated CZ, gentamicin, and rifampin. | |
Abbreviations: AV, arteriovenous; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CZ, cefazolin; DM2, type 2 diabetes mellitus; ESRD, end-stage renal disease; ETP, ertapenem; F, female; HbA1C, hemoglobin A1C; ICU, intensive care unit; IL-10, interleukin 10; IVDU, intravenous drug use; M, male; MSSA, methicillin-susceptible Staphylococcus aureus; PIP-TAZ, piperacillin-tazobactam.
aPatient eloped after 5 days, despite positive blood cultures, but returned to the hospital on day 7 with ongoing bacteremia.
bFollow-up blood cultures were obtained 3 days after CZ plus ETP salvage therapy.
Bacterial Strains and In Vitro Assays
MSSA isolates (isolated and identified by the clinical microbiology laboratory via routine workup of clinical specimens via MicroScan) from the initial blood culture were obtained from 6 clinical cases and evaluated for in vitro susceptibility to cefazolin, ertapenem, and nafcillin under both standard Clinical and Laboratory Standards Institute (105 colony-forming units [CFU]/mL) and high-inoculum (107 CFU/mL) conditions using both standard Mueller-Hinton broth (MHB) bacteriological media and Roswell Park Memorial Institute (RPMI) physiological cell culture media supplemented with 5% Luria-Bertani (LB) broth [9]. Checkerboard assays were also performed using standard and high inoculums in MHB [10]. For kill curve studies, bacteria were grown overnight in Todd-Hewitt broth at 37°C with shaking to stationary phase and diluted in MHB or RPMI + 5% LB to an optical density at 600 nm = 0.40. Cultures were diluted in MHB or RPMI + 5% LB to an initial inoculum of 1 × 107 CFU/mL. Antibiotics were added at one-fourth the MIC, and tubes were placed in a shaking incubator at 37°C. Aliquots were collected at 6 hours and 24 hours and serially diluted for CFU enumeration. These data were collected from at least 3 biological replicates performed in at least technical triplicate. All antibiotics were purchased from the Sharp Memorial Hospital pharmacy (San Diego, California), supplied as vials available for clinical use and administration to patients.
Disk diffusion synergy assays between cefazolin and ertapenem were performed as previously described [8]. In brief, a bacterial suspension of 0.5 McFarland (108 CFU/mL) was streaked as a lawn on brain-heart infusion agar plates. A cefazolin or ertapenem disk was placed in the center of the plate and was replaced with a fresh cefazolin disk 1 hour later. Diameter of the zone of inhibition was measured after incubation at 37°C for 24 hours. Synergy was defined as >3-mm increase in zone size when sequential disks of different agents were used, as compared to a single antimicrobial disk. Disks were purchased from Hardy Diagnostics (Santa Maria, California).
Rat Aortic Valve Endocarditis Model
The well-characterized MSSA strain TX0117 (harboring a type A β-lactamase and exhibiting a cefazolin inoculum effect) [4, 11] was studied in an established model of endocarditis using male Sprague-Dawley rats (weight ~200 g) [4, 11, 12]. Thirty hours after bacterial inoculation, therapy was started with ertapenem 30 mg/kg SC every 8 hours, cefazolin 50 mg/kg intramuscularly every 8 hours, or a combination of cefazolin plus ertapenem at the above doses and intervals. Dosages were selected based on prior use of this model to assess antimicrobial therapeutics (authors K. V. S., B. E. M.). Antibiotic therapy was administered for 3 days, animals were killed approximately 15 hours after the last antibiotic dose, and vegetations formed on the aortic valve and surrounding tissues were aseptically removed, weighed, and homogenized in 1 mL of 0.9% saline solution. Sequential dilutions of the homogenized tissues were carried out and subsequently, the entire volume of each dilution (including the undiluted sample) was plated onto BHI agar. The geometric mean log10 CFU/g and standard deviations were calculated from colonies recovered from vegetations, and treatment groups were compared to untreated controls. Animals were included in the final analysis only if the catheters were found across the aortic valve in the left ventricle, and only rats that survived beyond the first 24 hours of therapy were included in the treatment group. The minimum detection limit of bacteria by this method was 10 CFU/g of tissue. Results were analyzed as previously described [4, 11, 12].
Statistical Analyses
Statistical analyses were performed using GraphPad Prism, version 7.0d. P values <.05 were considered significant.
RESULTS
A total of 11 cases of persistent MSSA bacteremia were successfully cleared with cefazolin plus ertapenem combination therapy (Table 1). All isolates were methicillin susceptible per the clinical microbiology laboratory. The first case listed in Table 1 is from the previous case report [8]. Median duration of bacteremia was 6 days (range, 4–9 days) while on antibiotics prior to cefazolin plus ertapenem salvage therapy. Infective endocarditis was definitively identified by echocardiography in 6 of these cases, including 2 cases of tricuspid valve endocarditis where cardiac vegetations were ≥2 cm in size. Remarkably, in these 2 cases, bacteremia cleared within 24 hours after the initiation of the salvage regimen. Among the 9 cases where blood cultures were drawn daily, bacteremia cleared within 24 hours in 8 cases (88%). In 2 cases, blood cultures were not obtained until day 3 of salvage therapy, so it was not possible to define the exact duration of bacteremia. While the patients included in this case report were not enrolled in a clinical trial for which outcome metrics were prespecified, all patients survived to hospital discharge.
In vitro assessment of cefazolin, ertapenem, and nafcillin activity for the 6 available isolates is shown in Table 2. Three of the 6 isolates exhibited a significant inoculum effect with cefazolin, with MIC >3 dilutions higher when susceptibility testing was done using 107 CFU/mL vs 105 CFU/mL (Table 2). The high-inoculum cefazolin MICs ranged from 8 mg/L to 32 mg/L. Nafcillin and ertapenem showed no inoculum effect.
Table 2.
In Vitro Studies in Mueller-Hinton Broth Under Low or High Inocula Against Nafcillin, Cefazolin, and Ertapenem From Methicillin-Susceptible Staphylococcus aureus Obtained From 6 Clinical Cases
| Case | MIC, mg/L | Checkerboard, FICI | Disk Diffusion, mm | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NAF | CZ | ETP | CZ + ETP | NAF + ETP | ETP | CZ | ETP→CZ | Δ | ||||||
| 105 | 107 | 105 | 107 | 105 | 107 | 105 | 107 | 105 | 107 | |||||
| 1 | 0.25 | 0.50 | 0.25 | 1 | 0.06 | 0.06 | 0.56 | 0.50 | 0.56 | 0.75 | 30 | 27 | 32 | +5 |
| 2 | 0.50 | 1 | 1 | 16 | 0.13 | 0.25 | 0.50 | 0.38 | 0.53 | 0.50 | 29 | 24 | 29 | +5 |
| 3 | 0.25 | 0.50 | 0.50 | 8 | 0.06 | 0.06 | 0.75 | 0.56 | 0.38 | 0.56 | 29 | 26 | 29 | +3 |
| 4 | 0.25 | 0.50 | 0.25 | 1 | 0.06 | 0.06 | 0.50 | 0.63 | 0.63 | 0.56 | 30 | 28 | 32 | +4 |
| 5 | 0.25 | 0.50 | 0.50 | 32 | 0.03 | 0.06 | 0.75 | 0.53 | 0.50 | 0.63 | 30 | 24 | 28 | +4 |
| 6 | 0.25 | 0.25 | 0.25 | 0.50 | 0.03 | 0.06 | 1 | 0.63 | 0.75 | 0.63 | 30 | 30 | 34 | +4 |
Data in bold indicate isolates that exhibited an inoculum effect with cefazolin based on MIC; or synergy between cefazolin or nafcillin + ertapenem based on checkerboard, FICI. FICIs were interpreted as follows: synergy, FICI of ≤0.50; additivity, FICI of >0.50 to ≤1.0; no interaction (indifference), FICI of >1 to ≤4; antagonism, FICI of >4. ETP→CZ: ertapenem disk placed for 1 hour, then replaced by CZ disk for overnight. This was compared to CZ where a blank disk was placed for an hour and replaced with CZ for overnight incubation. The difference (∆in zone size between CZ and ETP→CZ was measured (mm) and synergy defined as >3 mm.
Abbreviations: CZ, cefazolin; ETP, ertapenem; FICI, fractional inhibitory concentration indices; MIC, minimum inhibitory concentration; NAF, nafcillin.
Checkerboard testing revealed general additivity with some synergy between ertapenem and cefazolin or nafcillin based on fractional inhibitory concentration index calculations (Table 2). Disk diffusion assays compared zones of inhibition using a cefazolin disk with or without agar priming by prior placement of an ertapenem disk for 1 hour (Table 2). All the isolates showed at least a 4-mm increase in cefazolin inhibition zone with ertapenem priming.
We and others have recently appreciated that susceptibility testing results obtained in bicarbonate-buffered bacteriological media or physiologically relevant tissue culture–based media can be more reflective of antibiotic activity in vivo [13–15]. Therefore, susceptibility testing for cefazolin, nafcillin, and ertapenem was also performed in RPMI media supplemented with 5% LB under both standard and high inoculum conditions (Table 3). For cefazolin and nafcillin, MICs obtained in RPMI + 5% LB under standard inoculum conditions were similar to results obtained in standard MHB. Isolates from cases 2 and 5 showed 8-fold decreased cefazolin MIC in RPMI + 5% LB compared to MHB when tested under high inoculum. Only the MSSA isolate from case 5, which had a very high cefazolin MIC (32 mg/L) in MHB and high bacterial inoculum, showed a significant inoculum effect in RPMI + 5% LB (cefazolin MIC 4 mg/L). However, much higher ertapenem MICs were seen for all of isolates under both standard and high inoculum testing in RPMI + 5% LB media. Using standard bacterial inocula, the ertapenem MICs observed in RPMI + 5% LB were 15–133 times higher than in MHB.
Table 3.
Minimum Inhibitory Concentration Under Low and High Inocula for Nafcillin, Cefazolin, and Ertapenem Across the 6 Clinical Isolates of Methicillin-Susceptible Staphylococcus aureus Used in This Study in Roswell Park Memorial Institute 1640 Tissue Culture Medium Supplemented With 5% Luria-Bertani Broth
| Case | MIC, mg/L | |||||
|---|---|---|---|---|---|---|
| Nafcillin | Cefazolin | Ertapenem | ||||
| 105 | 107 | 105 | 107 | 105 | 107 | |
| 1 | 0.13 | 0.25 | 0.25 | 1 | 4 | 2 |
| 2 | 0.25 | 0.50 | 0.50 | 2 | 2 | 4 |
| 3 | 0.25 | 0.50 | 1 | 4 | 4 | 2 |
| 4 | 0.25 | 0.25 | 0.50 | 1 | 2 | 2 |
| 5 | 0.13 | 0.13 | 0.25 | 4 | 4 | 4 |
| 6 | 0.13 | 0.25 | 0.25 | 1 | 4 | 1 |
Data in bold indicate isolates that exhibited an inoculum effect with cefazolin based on MIC.
Abbreviation: MIC, minimum inhibitory concentration.
To simulate the most challenging high-inoculum MSSA infections, kill curves were performed at a starting inoculum of 107 CFU/mL in addition to standard inoculum (105 CFU/mL) with ertapenem and cefazolin, alone or in combination, against clinical strains from cases 1–6 listed in Table 1. Results are shown in Supplementary Figure 1, with data from experiments in MHB on the left and those done in RPMI + 5% LB on the right. In both MHB and RPMI + 5% LB media, ertapenem and cefazolin provided heterogenous results across the 6 strains, with some strains showing synerg (≥2 log10 killing of the combination as compared to the most active antibiotic alone) [16] at the 24-hour time point while others did not. Most strains showed regrowth at 24 hours. Two of the strains were also examined in a similar fashion using ertapenem and nafcillin, alone or in combination in both MHB and RPMI + 5% LB (Supplementary Figure 2). Both strains showed synergism with the combination of ertapenem plus nafcillin in RPMI + 5% LB, with approximately 2 log10 killing at 24 hours but only at high inoculums (107 CFU/mL).
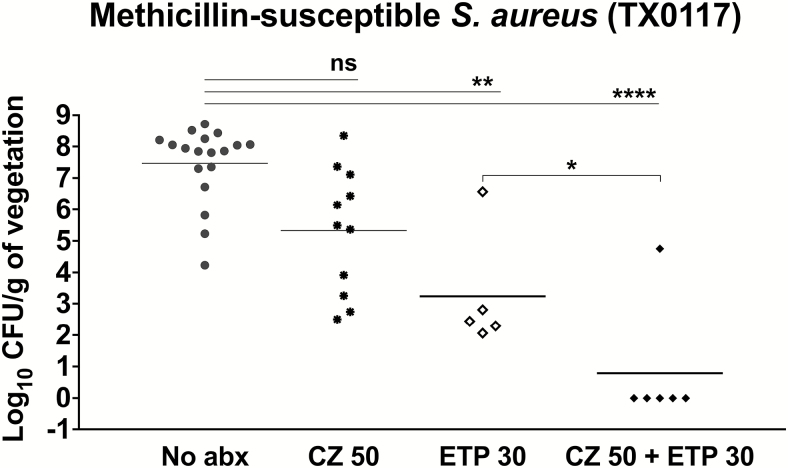
The results of cefazolin and ertapenem, alone and in combination, in the endocarditis model are shown in Figure 1. In the presence of ertapenem, the bacterial inoculum in all but 1 valve was reduced below the limit of detection. The addition of cefazolin to ertapenem trended toward increased activity (P < .0001) compared to cefazolin (P = .0591) or ertapenem (P = .005) alone. Additionally, the results achieved statistical significance when the ertapenem treatment group was compared to the cefazolin plus ertapenem condition (P < .05).
Figure 1.
Efficacy of antibiotic therapy in a rat endocarditis model of infection with methicillin-susceptible Staphylococcus aureus TX0117. The results of therapy with cefazolin, ertapenem, and cefazolin-ertapenem for TX0117-infected rats are shown. Horizontal bars represent the geometric mean colony-forming unit titers. No antibiotics, filled circles; cefazolin, filled asterisks; ertapenem, open diamonds; cefazolin + ertapenem, closed diamonds. *P < .05, by 2-tailed Mann-Whitney test. **P = .005, ****P < .0001, by Kruskal-Wallis 1-way analysis of variance. Abbreviations: abx, antibiotics; CFU, colony-forming units; CZ, cefazolin; ETP, ertapenem; ns, not significant.
DISCUSSION
Cefazolin has been increasingly used and recommended for treatment of serious MSSA infections such as bacteremia, endocarditis, and osteomyelitis. In fact, recent studies have shown increased tolerability and perhaps better outcomes in patients receiving cefazolin compared to classical antistaphylococcal β-lactams such as oxacillin and nafcillin [17–19]. These favorable data for cefazolin are somewhat limited as they were retrospectively collected and, therefore, vulnerable to bias (eg, higher-risk patients may be biased toward receiving antistaphylococcal β-lactams over cefazolin). Yet, they do speak to the more favorable drug tolerability of cefazolin, particularly with longer treatment durations of >4 weeks called upon by these more serious infections, especially in the elderly. For example, myelosuppression and acute kidney injury are much less common with cefazolin than with the classical antistaphylococcal β-lactams [17–19]. However, the inferior activity of cefazolin under high inoculum conditions against some MSSA (eg, type A β-lactamase–producing strains) raises concern for treatment failure, which has been documented in the literature [4–7]. High-level population data may not highlight individual cases caused by MSSA exhibiting the cefazolin inoculum effect if such isolates are not very common, and recent studies suggest that these isolates may be common in some settings but quite rare in other hospital centers [20–23]. Staphylococcus aureus inoculum effects have recently been shown for additional β-lactam drugs, including β-lactam/β-lactamase inhibitor combinations [24]. Suboptimal antimicrobial therapy against MSSA may have catastrophic clinical consequences in very severe infections such as endocarditis or epidural abscess. It is noteworthy that 3 of the 6 MSSA isolates examined in this study showed a cefazolin inoculum effect, although only case 5 showed a cefazolin inoculum effect in RPMI + 5% LB media. We highlight that the patient from this case experienced overt clinical failure requiring readmission to the hospital a few days after ertapenem was discontinued and cefazolin remained as monotherapy. Whether clinically significant cefazolin inoculum effects warrant testing in physiological media such as RPMI is a potential topic for future study, especially if cefazolin comes to replace classical antistaphylococcal β-lactams as the treatment of choice for complex MSSA infections.
In this study, we have shown that cefazolin plus ertapenem combination salvage therapy resulted in rapid MSSA bacteremia clearance in patients failing standard monotherapy, even in cases with large-burden endovascular infections on echocardiogram. A modest synergy or additivity of cefazolin plus ertapenem against MSSA was observed in vitro utilizing both bacteriologic (MHB) and physiologic (RPMI) media. Considerable discordance was seen between in vitro synergy testing by the disk diffusion, checkerboard, and time-kill assays, overall raising questions as to how clinically relevant results of these assays are in assessing what appears to be a strong synergy between cefazolin and ertapenem in vivo, corroborated by significant synergy in the rat model of endocarditis. Based on these cumulative results, cefazolin plus ertapenem appears to offer a viable salvage regimen option in patients with MSSA bacteremia refractory to standard β-lactam therapy, provided appropriate surgical source control has been performed. While the clinical data and the rat endocarditis model data obtained with 1 bacterial strain support the hypothesis that ertapenem plus cefazolin combination has greater efficacy, the in vitro data were less convincing, with limited correlation between strains or assays.
The initial rationale for selecting this combination was to provide therapy with 2 β-lactam antibiotics with complementary penicillin binding protein (PBP)–binding proclivities, thus simultaneously targeting multiple steps in cell wall synthesis to provide enhanced killing [25]. Specifically, carbapenem antibiotics have exceptional affinity to the essential PBP of S. aureus, PBP1, exceeding even that of the antistaphylococcal β-lactams [25, 26]. This would complement the relative PBP2 proclivity of cefazolin [27]. A similar rationale has been the basis for use of ampicillin plus ceftriaxone to treat Enterococcus faecalis endocarditis and, due to its better tolerability, is phasing out toxic aminoglycoside therapy in this disease [28]. Our in vitro studies demonstrate additivity or synergy, without evidence of antagonism against the isolates tested. However, clearing bacteremia in <24 hours in patients with cardiac vegetations >2 cm in size far exceeds the predicted expectations from the in vitro studies, suggesting that additional factors may be involved that require further study.
One possibility for the profound effect in vivo may be the sensitization of MSSA exposed to both antibiotics to the innate immune system. We illustrated this phenomenon in our prior study, wherein MSSA from case 1 was more effectively killed by the human cationic host defense peptide, cathelicidin LL-37, or by neutrophils that produce many antimicrobial factors, when exposed to sub-MIC concentrations of both cefazolin and ertapenem compared to either drug alone [8]. Another possibility is that very potent interference of PBP1, the only essential PBP in S. aureus, by the addition of ertapenem may surpass some cellular viability threshold that cannot be compensated by the other PBPs, particularly if another β-lactam is also interfering with their functions. Finally, our extensive review of the literature has revealed that expression of PBP2, the primary target for cefazolin, is diminished in the presence of neutrophils [27]. Thus, our assessment of antimicrobial activity in artificially contrived bacterial media may be ill-equipped to examine dynamic changes to cell wall that occur in vivo. In reference to the cefazolin-ertapenem interaction, ertapenem may serve to “rescue” the relatively attenuated activity of cefazolin that may be occurring in microenvironments such as bacterial endocarditis vegetations. More studies will be needed to examine the relative activities of cefazolin in the presence of components of innate immunity present in vegetations.
In summary, we present a case series of consecutively treated patients with refractory MSSA bacteremia who achieved prompt bacteremia clearance with cefazolin plus ertapenem combination therapy. Synergy or additivity were observed in vitro between cefazolin and ertapenem against MSSA available from 6 of the treated patients and corroborated by studies in a rat endocarditis model. Based on the potent effects observed in vivo beyond what is predicted by in vitro assays, we hypothesize that cooperativity with innate immunity in vivo may also aid in bacterial killing when cefazolin and ertapenem are used together, as previously described [8]. Ertapenem with either cefazolin or other anti-staphylococcal β-lactams should be more extensively evaluated in the clinical setting to establish the role of these combination regimens in refractory cases of MSSA bacteremia and endocarditis.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This research was supported by the National Institutes of Health: the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; Pediatric Scientist Development Program Fellowship K12-HD000850 to E. R. U.) and the National Institute of Allergy and Infectious Diseases (grant numbers U01-AI124316-01 and U54-HD090259 to V. N. and G. S.).
Potential conflicts of interest. G. S. has received speaking honoraria from Allergan and Melinta Pharmaceuticals, and consulting fees from Allergan and Paratek Pharmaceuticals. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Fowler VG Jr, Olsen MK, Corey GR, et al. . Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003; 163:2066–72. [DOI] [PubMed] [Google Scholar]
- 2. Naber CK. Staphylococcus aureus bacteremia: epidemiology, pathophysiology, and management strategies. Clin Infect Dis 2009; 48(Suppl 4):S231–7. [DOI] [PubMed] [Google Scholar]
- 3. Holland TL, Arnold C, Fowler VG Jr. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA 2014; 312:1330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nannini EC, Singh KV, Murray BE. Relapse of type A beta-lactamase-producing Staphylococcus aureus native valve endocarditis during cefazolin therapy: revisiting the issue. Clin Infect Dis 2003; 37:1194–8. [DOI] [PubMed] [Google Scholar]
- 5. Bryant RE, Alford RH. Unsuccessful treatment of staphylococcal endocarditis with cefazolin. JAMA 1977; 237:569–70. [PubMed] [Google Scholar]
- 6. Quinn EL, Pohlod D, Madhavan T, Burch K, Fisher E, Cox F. Clinical experiences with cefazolin and other cephalosporins in bacterial endocarditis. J Infect Dis 1973; 128(Suppl):S386–9. [DOI] [PubMed] [Google Scholar]
- 7. Fernandez-Guerrero ML, de Gorgolas M. Cefazolin therapy for Staphylococcus aureus bacteremia. Clin Infect Dis 2005; 41:127. [DOI] [PubMed] [Google Scholar]
- 8. Sakoulas G, Olson J, Yim J, et al. . Cefazolin and ertapenem, a synergistic combination used to clear persistent Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2016; 60:6609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 26th ed. CLSI Supplement M100S. Wayne, PA: CLSI, 2016. [Google Scholar]
- 10. Moody J, Knapp C.. Tests to assess bactericidal activity, in clinical microbiology procedures handbook. In: Garcia LS, ed. Washington, DC: ASM Press, 2010. [Google Scholar]
- 11. Nannini EC, Singh KV, Arias CA, Murray BE. In vivo effects of cefazolin, daptomycin, and nafcillin in experimental endocarditis with a methicillin-susceptible Staphylococcus aureus strain showing an inoculum effect against cefazolin. Antimicrob Agents Chemother 2013; 57:4276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singh KV, Tran TT, Nannini EC, Tam VH, Arias CA, Murray BE. Efficacy of ceftaroline against methicillin-susceptible Staphylococcus aureus exhibiting the cefazolin high-inoculum effect in a rat model of endocarditis. Antimicrob Agents Chemother 2017; 61. doi:10.1128/AAC.00324-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin L, Nonejuie P, Munguia J, et al. . Azithromycin synergizes with cationic antimicrobial peptides to exert bactericidal and therapeutic activity against highly multidrug-resistant gram-negative bacterial pathogens. EBioMedicine 2015; 2:690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kumaraswamy M, Lin L, Olson J, et al. . Standard susceptibility testing overlooks potent azithromycin activity and cationic peptide synergy against MDR Stenotrophomonas maltophilia. J Antimicrob Chemother 2016; 71:1264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ersoy SC, Heithoff DM, Barnes L 5th, et al. . Correcting a fundamental flaw in the paradigm for antimicrobial susceptibility testing. EBioMedicine 2017; 20:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doern CD. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J Clin Microbiol 2014; 52:4124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li J, Echevarria KL, Hughes DW, Cadena JA, Bowling JE, Lewis JS 2nd. Comparison of cefazolin versus oxacillin for treatment of complicated bacteremia caused by methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 2014; 58:5117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McDanel JS, Roghmann MC, Perencevich EN, et al. . Comparative effectiveness of cefazolin versus nafcillin or oxacillin for treatment of methicillin-susceptible Staphylococcus aureus infections complicated by bacteremia: a nationwide cohort study. Clin Infect Dis 2017; 65:100–6. [DOI] [PubMed] [Google Scholar]
- 19. Weis S, Kesselmeier M, Davis JS, et al. . Cefazolin versus anti-staphylococcal penicillins for the treatment of patients with Staphylococcus aureus bacteraemia. Clin Microbiol Infect 2019; 25:818–27. [DOI] [PubMed] [Google Scholar]
- 20. Nannini EC, Stryjewski ME, Singh KV, et al. . Inoculum effect with cefazolin among clinical isolates of methicillin-susceptible Staphylococcus aureus: frequency and possible cause of cefazolin treatment failure. Antimicrob Agents Chemother 2009; 53:3437–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang SK, Gilchrist A, Loukitcheva A, et al. . Prevalence of a cefazolin inoculum effect associated with blaZ gene types among methicillin-susceptible Staphylococcus aureus isolates from four major medical centers in Chicago. Antimicrob Agents Chemother 2018; 62. doi:10.1128/AAC.00382-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chong YP, Park SJ, Kim ES, et al. . Prevalence of blaZ gene types and the cefazolin inoculum effect among methicillin-susceptible Staphylococcus aureus blood isolates and their association with multilocus sequence types and clinical outcome. Eur J Clin Microbiol Infect Dis 2015; 34:349–55. [DOI] [PubMed] [Google Scholar]
- 23. Rincón S, Reyes J, Carvajal LP, et al. . Cefazolin high-inoculum effect in methicillin-susceptible Staphylococcus aureus from South American hospitals. J Antimicrob Chemother 2013; 68:2773–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Song KH, Jung SI, Lee S, et al. . Korea Infectious Diseases (KIND) Study Group Inoculum effect of methicillin-susceptible Staphylococcus aureus against broad-spectrum beta-lactam antibiotics. Eur J Clin Microbiol Infect Dis 2019; 38:67–74. [DOI] [PubMed] [Google Scholar]
- 25. Chambers HF, Sachdeva M. Binding of beta-lactam antibiotics to penicillin-binding proteins in methicillin-resistant Staphylococcus aureus. J Infect Dis 1990; 161:1170–6. [DOI] [PubMed] [Google Scholar]
- 26. Dumitrescu O, Choudhury P, Boisset S, et al. . Beta-lactams interfering with PBP1 induce Panton-Valentine leukocidin expression by triggering sarA and rot global regulators of Staphylococcus aureus. Antimicrob Agents Chemother 2011; 55:3261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bamberger DM, Herndon BL, Fitch J, Florkowski A, Parkhurst V. Effects of neutrophils on cefazolin activity and penicillin-binding proteins in Staphylococcus aureus abscesses. Antimicrob Agents Chemother 2002; 46:2878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pericas JM, Cervera C, del Rio A, et al. . Hospital Clinic Endocarditis Study Group Changes in the treatment of Enterococcus faecalis infective endocarditis in Spain in the last 15 years: from ampicillin plus gentamicin to ampicillin plus ceftriaxone. Clin Microbiol Infect 2014; 20:O1075–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.