Abstract
Apoptosis, necroptosis and pyroptosis represent three major regulated cell death modalities. Apoptosis features cell shrinkage, nuclear fragmentation and cytoplasm-blebbing. Necroptosis and pyroptosis exhibit osmotic imbalances in the cell accompanied by early membrane ruptures, which morphologically resembles necrosis. Importantly, these two lytic cell death forms facilitate the release of damage associated molecular patterns into the extracellular space leading to inflammatory response. Whereas, during apoptosis, the membrane integrity is preserved and the apoptotic cell is removed by neighbouring cells ensuring the avoidance of immune-stimulation. Viruses comprise a versatile group of intracellular pathogens, which elicit various strategies to infect and to propagate. Viruses are recognized by a myriad of pathogen recognition receptors in the human cells, which consequently lead to activation of the immune system and in certain circumstances cell-autonomous cell death. Importantly, the long-standing view that a cell death inducing capacity of a virus is equal to its pathogenic potential seems to be only partially valid. The altruistic cell death of an infected cell may serve the whole organism by ultimately curbing the way of virus manufacturing. In fact, several viruses express “anti-cell death” proteins to avoid this viral-defence mechanism. Conversely, some viruses hijack cell death pathways to selectively destroy cell populations in order to compromise the immune system of the host. This review discusses the pros and cons of virus induced cell death from the perspective of the host cells and attempts to provide a comprehensive overview of the complex network of cell death signalling in virus infection.
Keywords: Cell death, Apoptosis, Necroptosis, Pyroptosis, Virus, Infection
1. Introduction
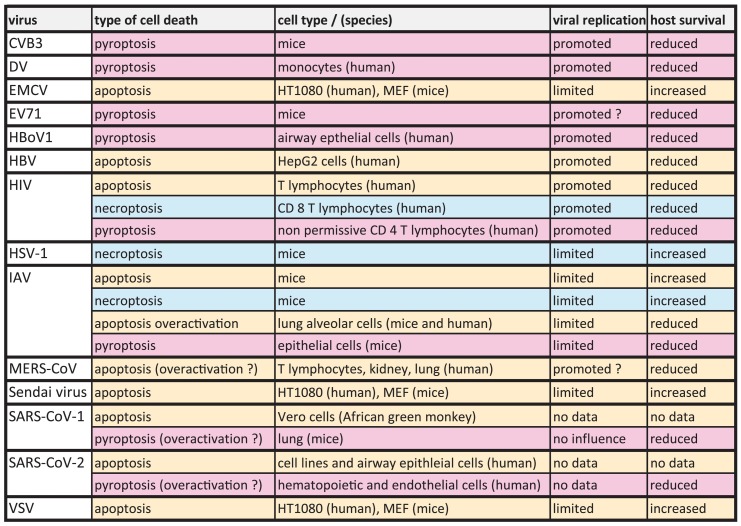
For a long time, a cell death inducing capacity of a virus was interpreted as the measure of its pathogenic potential. Nevertheless, viruses are obligatory intracellular pathogens, thus their propagation is entirely dependent on the intracellular environment of the host cells. Therefore, the question is legitimate: Can cell death paradoxically protect from virus infection? (Table 1 .). In a single cell level, the emergence of immediate cell death in response to infection ultimately blocks the way of virus propagation and therefore protects the rest of the cell population from an increasing viral burden. The strongest argument for this theory comes from experimental observations on viruses which, in fact, actively evade cell death pathways by encoding viral “anti-death” proteins (in detail see Sections 2.4, 3.3 and Table 2 .). If, however, the relation of cell death and virus infection is scrutinized at organism level, the answer for the question is more complex, particularly, since viruses represent a broad group of pathogens with different tissue-tropisms and various strategies to infect and to replicate. In general, the interplay of three key factors can determine the final outcome of cell death in virus infection: timing, immunogenic capacity and tissue specificity of the cell destruction. For instance, manifestation of a cell death with delayed kinetics can allow sufficient time for virus replication and therefore might not deliver any benefit for the host, or selective killing of immune cells results in attenuated immune-response, which is described in the course of human immunodeficiency virus (HIV) infection of T lymphocytes [1]. Furthermore, even a virus propagation-limiting cell death is considered detrimental in tissues with limited renewing potential (e.g. central nervous system) or when the function of the tissue is compromised.
Table 1.
The outcome of cell death on viral replication.
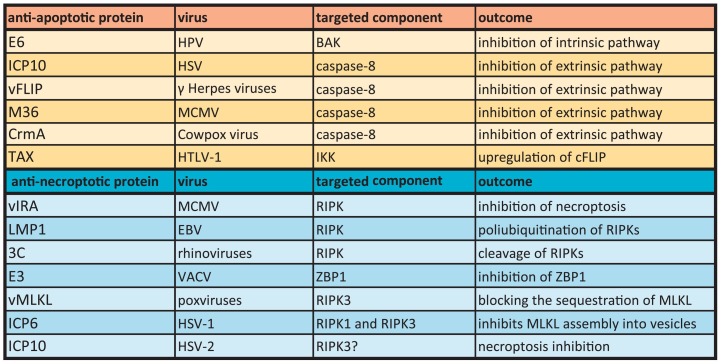
Table 2.
Virally encoded anti-apoptotic and anti-necroptotic proteins.
If we assume that a given virus infection selectively triggers cell death pathways, then it is expected that the cells exhibit specific “sensors” to recognize this particular event. In fact, detection of viruses in mammalian cells is accomplished by the recognition of molecular elements which are decoded as foreign-to-the-organism, collectively termed as pathogen associated molecular patterns (PAMPs). Two distinct mechanisms can be identified in the context of PAMPs and cell death. First, PAMPs are recognized by so called pathogen recognition receptors (PRRs) [2], which in turn induce pathways leading to immune response and -in certain circumstances- indirectly to cell demise [1]. Second, PAMPs can also initiate cell-autonomous death signalling pathways.
In this article, the machinery of pathogen recognition is reviewed in the aspect of cell death signalling and the major regulated cell death modalities, including apoptosis, necroptosis and pyroptosis are discussed in detail, which can emerge during a viral insult. Importantly, regulated cell death forms differ in their inflammatory potential, as apoptosis represent an anti-inflammatory cell death form, whereas necroptosis and pyroptosis may render immune stimulation more efficiently.
2. Apoptosis
2.1. Apoptotic core machinery
Apoptosis is a widely studied regulated cell death form, which has been first described by Kerr and colleagues by the help of electron microscopic techniques in the 60´s of the last century [3]. Morphologically, apoptosis exhibits cell shrinkage, condensation and fragmentation of the nucleus. Immunologically important features of apoptosis include preserved membrane integrity of the apoptotic cells and engulfment of the dying cells by neighbouring phagocytes [4]. This special “immunologically silent” characteristic enables apoptosis to play a central role in physiological processes such as development of organs and maturation of immune cells. Caspases take a central stage in apoptosis regulation. Functionally, caspases are divided into three subgroups: inflammatory, apoptotic-initiator and apoptotic-executioner caspases [5]. Initiator caspases are structurally distinguished by their large pro-domain, which enables them to be recruited into high-molecular-weight protein complexes in response to an apoptotic stimulus [6]. Intrinsic and extrinsic pathways represent the two main pathways of apoptosis initiation. Intrinsic pathway is triggered by intracellular stress signals such as genotoxic stress, whereas extrinsic pathway is initiated by the ligation of transmembrane receptors of the tumor necrosis factor (TNF) superfamily, commonly termed death receptors (DR). Stimulation of the intrinsic pathway leads to conformational changes of pro-apoptotic members of the B cell lymphoma-2 (BCL-2) protein family: BCL-2-associated X protein (BAX) and BCL-2-antagonistic killer (BAK). Consequently, BAX and BAK translocate, oligomerize and form large pores throughout the mitochondrial membrane [7,8] leading to mitochondrial outer membrane permeabilization (MOMP) and resulting in the release of cytochrome-C [9]. The assembly of apoptosome in the cytosol, a complex containing cytochrome C and apoptotic protease activating factor-1 (APAF-1), culminates in the recruitment of caspase-9 [9]. In the extrinsic pathway, the ligation of DRs leads to the recruitment of initiator caspases-8 and -10 in a high molecular weight complex termed death inducing signalling complex (DISC). The recruitment and oligomerization of initiator caspases in both pathways enables proximity-driven self-cleavage and activation [10]. As a consequence, the activation of initiator caspases results in the cleavage and activation of effector caspases (caspase-3, -6 and -7), and finally activated effector caspases selectively process their substrates. The cleavage of them results in a series of events which finally leads to apoptosis [11].
2.2. Immune response-driven cell-killing mechanisms
Cytotoxic T cells (CTL) play crucial role in the adaptive immune response and natural killer (NK) cells exert similar functions in the innate immune response given to viral infection. In vivo studies by employing reporter viruses demonstrate that one single CTL is capable to kill up to 16 infected cells per day [12]. It is noteworthy that CTLs exhibit distinct cell killing capacity in different tissue types. For instance, Herpes simplex and Vaccinia virus infected monocytes and macrophages are killed by CTLs, whereas epithelial cells are less susceptible to CTL mediated cell death mechanisms [13,14]. CTL and also NK cell driven cell killing is dependent on cell-cell contacts and involves the rapid secretion of cytotoxic perforin and granzymes [15] and the slower death receptor, Fas (also: CD95)-driven apoptotic pathway (Fig. 1 .). Granzymes are a family of serine proteases that are produced and stored in lytic granules inside the CTLs and NK cells. A well-studied member of this family, Granzyme-B can enter the target cells either via newly formed trans-membrane perforin-pores [15] or by endocytosis. Subsequently, granzyme-B induces mitochondrial apoptosis by performing cleavage of the BCL-2 homology domain-3 (BH3)-only protein, BH3 interacting domain death agonist (BID), which then leads to BAX/BAK-mediated MOMP and the initiation of the caspase-9-driven apoptotic pathway [16]. CTLs express Fas ligands (FasL) on their surface and the trimerized ligands are capable to bind the death receptor Fas, which is present on the cell surface of the target cells. The ligation of the DRs results in the recruitment of the adaptor protein, Fas-associated protein with death domain (FADD), leading to recruitment of initiator caspases with a death domain (caspase-8/-10). Interestingly the sensitivity towards DR-mediated cell killing is also selectively regulated on the host side, since in response to Influenza A virus (IAV) and Dengue virus infection, the upregulation of the elements of Fas pathway is observed. Furthermore, IAV infection leads to downregulation of the anti-apoptotic cellular caspase-8 and FADD-like apoptosis regulator (cFLIP) [17], which if highly expressed can form inactive hetero-dimers with caspase-8. Surprisingly, the replication of IAV can be amplified by overexpression of pro-apoptotic genes and is blocked by the upregulation of anti-apoptotic genes in human lung epithelial cell line [18], which indicates a virus-promoting role for apoptosis in response to IAV in lung cells. Along these lines, the viral protein Hepatitis B virus x (HBx) sensitizes host cells towards TNF driven apoptosis by interfering the anti-apoptotic action of cFLIP [19]. Increasing body of literature supports the relevance of HIV induced apoptosis. In infected individuals, the progressive depletion of CD4 T lymphocytes is observed over the period of 5–10 years, which leads to opportunistic infections and malignancies. Several HIV viral proteins exert pro-apoptotic or apoptosis sensitizing properties by increasing the expression of death ligands and DRs, including the envelope protein glycoprotein 120 and the transactivator of transcription (Tat) (reviewed in detail elsewhere: [20]). Interestingly, the protease of HIV, encoded by the pol gene is capable to directly process caspase-8 in order to produce a catalytically active caspase leading to BAK activation and mitochondrial apoptosis [21,22].
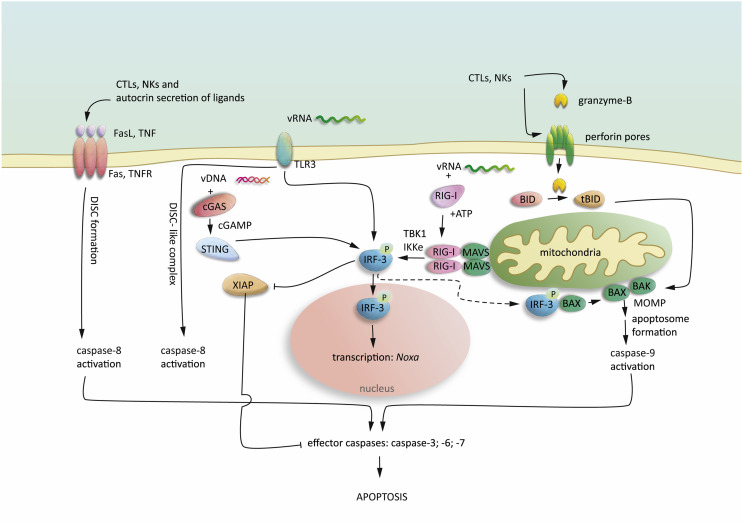
Fig. 1.
Apoptosis signalling in virus infection.
Virus infection leads to immune-stimulation and activation of CTLs and NK cells. CTLs and NK cells release cytotoxic perforin and granzyme-B. Granzyme-B enters the cells via the transmembrane perforin pores and results in BID cleavage, leading to BAX and BAK activation and MOMP-driven apoptosome formation and caspase-9 activation. Stimulation of death receptors (Fas and TNFR) initiate caspase-8 activation by the formation of death inducing signalling complex (DISC). Intracellular viral RNA (vRNA) is recognized by RIG-I, leading to ATP-dependent conformational changes and recruitment of MAVS oligomers, which in turn lead to the recruitment of TBK-1 and IKKε, resulting in phosphorylation of IRF-3. Extracellular vRNA is detected by TLR3 leading to IRF-3 activation. vDNA in the cytosol is recognized by cGAS, resulting in cGAMP production and activation of STING, which in turn activates IRF-3. Once IRF-3 is activated can translocate into the nucleus and induce transcriptional pathways leading to anti-viral response and apoptosis. Moreover, RIG-I activation promotes XIAP degradation and activation of pro-apoptotic BAX.
2.3. Virus infection-induced apoptosis by viral RNA and DNA fragments
Apart from the significant role of immune response-driven cell death, it has been observed that activation of PRRs could lead to direct -cell autonomous- apoptosis initiation. The group of RNA viruses (also termed: Riboviruses) comprises diverse pathogens that possess either double stranded RNA (dsRNA) (e.g.: Rotaviruses) or single stranded RNA (e.g.: Filoviruses) as genetic material. Since the spatial distribution and also the structure of a viral RNA (vRNA) can be distinct from that of the host RNA, thus vRNAs fit well in the definition of PAMPs. For instance, vRNAs are often di or tri-phosphorylated and they lack a 7-methylguanosine cap [23]. Detection of vRNAs takes place by different PRRs depending on the localization of the vRNA fragments.
Intracellular dsRNA fragments which are produced during virus replication are recognized in the cytosol by two caspase recruitment domain (CARD) containing RNA helicases: retinoic acid inducible gene-I (RIG-I) and melanoma differentiation-associated gene-5 (MDA-5) [24]. RIG-I activation by vRNAs leads to ATP-dependent conformational changes and enables tetramer formation [25], which via CARD-CARD homotypic interactions results in the recruitment of mitochondrial antiviral-signalling (MAVS), an adaptor protein associated with the mitochondrial membrane (Fig. 1.). At this point MAVS forms aggregates providing scaffold for multiple binding partners [26] and can bifurcate into two main pathways leading to activation of transcription factors interferon regulatory factor-3 (IRF-3) and nuclear factor kappa-light-chain-enhancer of activated B cells (NFᴋB) [27]. In the IRF-3 branch of the RIG-I pathway, TNFR associated factor-3 (TRAF-3) interacts with MAVS, which results in the recruitment of a protein complex comprising TRAF family member-associated NFkB activator (TANK), NFkB essential modulator (NEMO), inhibitor of kappa B kinase-ε (IKKɛ) and TANK binding kinase-1 (TBK1) and subsequent phosphorylation, dimerization and translocation to the nucleus of both IRF-3 and IRF-7 [28] (Fig. 1). Induction of IRF-3 primarily leads to the production of immunomodulatory molecules as Type I and III interferons (IFNs), which then renders the activation of CTLs, NK cells and initiates targeted cell-killing as discussed earlier (Section 2.2). Nonetheless, this process requires the external intervention of immune cells.
In contrast, studies on cell lines highlight the existence of direct death-inducing mechanisms. As for instance, IAV infected human epithelial cell lines undergo caspase-dependent cell death, which is prevented by genetic depletion of RIG-I [29]. In Sendai virus infection, the lack of IRF-3 leads to the loss of virus induced apoptosis sensitivity of the cells [30]. Analogously, Semliki forest virus infection leads to MAVS induction and caspase-8 activation [31]. RIG-I signalling leads to the expression of IFN-stimulated genes (ISGs) and this induces the intrinsic apoptosis pathway [32]. How exactly this apoptotic process is mediated is still poorly understood, however some studies provide hints about the possible mechanisms concerned, as for example the upregulation or activation of certain pro-apoptotic elements in a RIG-I dependent manner. Naturally occurring Vaccinia virus expresses a BCL-2 analogue anti-apoptotic protein called F1L. In contrast, infection with a genetically engineered Vaccinia virus strain lacking the F1L triggers IRF-3 activation and expression of the pro-apoptotic Noxa (from latin: danger) in monocytes and macrophages [33]. One work highlights the transcriptionally-independent function of IRF-3 in inducing the mitochondrial translocation of pro-apoptotic BAX and the initiation of intrinsic apoptosis [34], yet the transcriptional function of IRF-3 remains the predominant feature in majority of the studies. Along with this, IRF-3 dependent degradation of X-linked inhibitor of apoptosis (XIAP) delivers a potential link to virus induced apoptosis. XIAP is a potent antagonist of caspase-3, -7 and -9 dependent apoptotic signalling. Sendai and Vesicular stomatitis virus (VSV) infection results in TBK1/IKKε-mediated phosphorylation of XIAP in vivo at Ser430, leading to auto-ubiquitination and proteasomal degradation of XIAP. Given the multiple (~30) binding partners of aggregated MAVS [28] and a CARD domain structure highly similar to that of CARD of caspases it can be speculated that MAVS in certain conditions might recruit caspases with a CARD domain (e.g.: caspase-9 and -2), however this has not been investigated yet.
Extracellular vRNA detection is accomplished by toll-like receptor-3 (TLR3), located in cytoplasm membranes and endosomal compartments [35]. TLR3 recruits IKK via the adaptor protein Toll/interleukin-1 receptor (TIR) and leads to the activation of transcription factor NFkB [36] and results in the induction of IRF-3. In addition, TLR3 stimulation by IAV can lead to the assembly of a DISC-like complex containing TRIF, RIPK1, FADD, cFLIP and caspase-8 (Fig. 1) [37,38]. In this setting, the concentration of anti-apoptotic cFLIP isoforms determine, whether caspase-8 acts as an apoptotic initiator, since the formation of cFLIP/caspase-8 heterodimers works against the apoptotic homodimer formation of caspase-8.
Viral DNA in the cytosol triggers also alarm signals, since the DNA in its normal condition resides exclusively in the cell nucleus. The cyclic GMP-AMP (cGAMP) synthase (cGAS) exerts DNA binding activity, and upon binding produces cGAMP leading to the activation of protein stimulator of interferon gene (STING) (Fig. 1). As a consequence, STING induces IRF-3 activation [39] [40] and intrinsic apoptosis [41]. It is expected that the release of mitochondrial DNA (mtDNA) into the cytosol have similar consequences and can unleash cGAS driven IRF-3 activation. Intriguingly, apoptotic caspases suppress the STING mediated Interferon production [42] and studies on mice demonstrate that apoptotic caspases can cleave anti-viral sensors, including cGAS, MAVS and IRF-3 in order to reduce type I IFN production, indicating that caspase-dependent apoptosis might also contribute to virus propagation [43], but also suggests a negative feedback loop in controlling IFN production.
The most of the PAMP detection mechanisms which lead to apoptosis culminate in IRF-3 activation highlighting the significance of IRF-3 in virus-induced apoptosis. Still, the mechanism, by which IRF-3 triggers cell death signalling pathways is only partially understood and the studies indicate a strong cell type specificity in the apoptosis sensitivity in response to viral PAMPs.
Z-RNA and z-DNA fragments, which are distinct from the B-structure of eukaryotic RNA and DNA are recognized by z-DNA/RNA binding protein-1 (ZBP1; also: DAI). In response to IAV infection, ZBP1 interacts with receptor interacting serine/threonine kinase 3 (RIPK3) [44] and the recruitment of RIPK3 can initiate necroptosis (described in chapter 3), and can serve as a scaffold for caspase-8 activation-driven apoptosis [45]. Intriguingly, the IAV triggered cell death signalling seems to play a unique role in controlling the viral burden, which is discussed in detail in chapter 3.2.
2.4. Anti-apoptotic proteins encoded by viruses
Human papillomavirus (HPV) is a double stranded DNA virus associated with cervical cancer developments in humans [46]. The early gene-coding region Protein 6 (E6) of HPV interferes with apoptosis signalling at the level of p53 and interacts with the pro-apoptotic BAK protein. This example well demonstrates that most of viral anti-apoptotic proteins are encoded by large DNA viruses, which replicate with slower kinetics than RNA viruses. Nevertheless, unlike HPV, a majority of these viruses produce proteins that counteract with the extrinsic, caspase-8 dependent apoptotic pathway (Table 2.). For example, the large subunit of the Herpes simplex virus (HSV) ribonucleotide reductase ICP10 exerts apoptosis inhibitory potential by directly interacting with caspase-8 [47,48]. Along the same line, viral FLIP (vFLIP), the viral counterpart of cFLIP encoded by Molluscum contagiosum and several gamma Herpes viruses, such as Kaposi's sarcoma-associated herpes virus (KSHV), forms inactive heterodimers with caspase-8, which leads to the blockade of extrinsic apoptosis [49]. The Mouse cytomegalovirus (MCMV) is the experimental model of the human CMV, which causes asymptomatic infections in human, yet leads to severe infections in individuals with compromised immune system. Along with the previous instances, the M36 protein encoded by the MCMV also blocks the caspase-8-driven extrinsic apoptosis pathway [50,51]. Furthermore, an anti-apoptotic product of the Cowpox virus, cytokine response modifier A (CrmA) functions by the inhibition of the activity of caspase-8 [52]. A further group of viral proteins can influence the expression of host anti-apoptotic proteins. The adult T-cell leukemia/lymphoma (ATL) is a malignancy of mature CD4 T cells in humans [53] and infection with the human T-cell leukemia virus type-1 (HTLV-1) is linked to the disease. The transactivator protein TAX of HTLV-1 binds to IKK and thereby facilitates the activation of NFkB and subsequently leads to the upregulation of c-FLIP expression [54], which then exhibits caspase-8 inhibiting activity.
3. Necroptosis
3.1. The classical pathway: TNFR-dependent necroptosis signalling
Necroptosis is a non-apoptotic regulated cell death modality which morphologically resembles necrosis. In general, necroptosis exhibits cytoplasm swelling and early membrane rupture and facilitates the release of immunomodulatory damage associate molecular patterns (DAMPs), for instance ATP and high mobility group protein B1 (HMGB1), into the extracellular space [55] [56]. Necroptosis initiation takes place upon TNFR ligation, which, however, primarily leads to NFkB activation via the assembly of so called complex-I, including adaptor proteins TNFRSF1A associated via death domain (TRADD), TRAF2, cellular IAP (cIAP) and ubiquitinated receptor interacting serine/threonine kinase 1 (RIPK1) [10]. The state of RIPK1 is a decisive factor, since ubiquitination, phosphorylation at Ser89 by protein kinase-A and —C, and phosphorylation at several sites by IKKs can all attenuate the cytotoxic potential of this scaffold protein (Fig. 2 .) [57,58]. Once, however, the NFkB wing of the pathway is inhibited TNFR ligation can lead to a pro-apoptotic FADD and caspase-8 containing complex formation and apoptosis initiation. In the early 2000´s it has been observed that inhibition of caspases did not result in expected cell death inhibition, instead it led to a necrotic-like cell demise, which is nowadays termed necroptosis [59] [55]. RIPK1 and 3 play central role in the regulation of necroptotic signalling pathways [60] and fully active caspase-8 can cleave both of them [61,62], thus inhibition of caspase-8 is an absolute prerequisite for the assembly of RIPK1-RIPK3 containing necrosome complex and necroptosis initiation (Fig. 2.). The assembly of necrosome leads to phosphorylation of pseudo-kinase mixed lineage kinase-like (MLKL) [63], in turn the phosphorylation-driven conformational changes of MLKL result in oligomerization and subsequent pore formation throughout the cytoplasm membrane accompanied by osmotic imbalances and membrane ruptures.
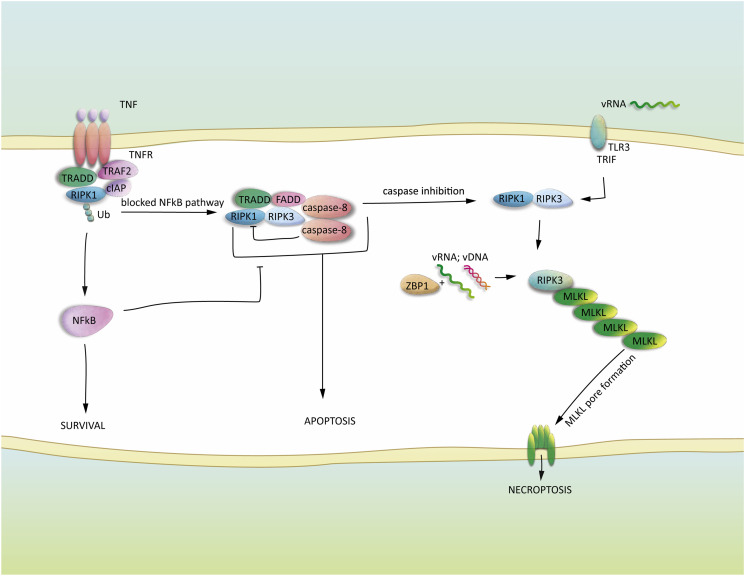
Fig. 2.
Necroptosis signalling in virus infection.
Ligation of TNFR recruits the NFkB activating pro-survival complex, containing ubiquitinated RIPK1. The formation of complex II, involving de-ubiquitinated RIPK1, enables activation of caspase-8 and apoptosis. Inactivation of caspase-8 leads to the assembly of intact RIPK1 and RIPK3 containing necrosome and phosphorylation of MLKL. vRNA and DNA binding by ZBP1 leads to RIPK3 recruitment and MLKL activation. vRNA detection by TLR3 results in RIPK1-RIPK3 necrosome formation and MLKL activation. The activation of MLKL in all three cases leads to oligomerization and transmembrane pore formation resulting in osmotic imbalances and the progression of necroptosis.
Necroptosis represents a lytic and immunogenic cell death modality, therefore it is less surprising that most of the viruses developed countermeasures to evade it. Nevertheless, sensitivity to virus-driven necroptosis varies among cell types and viruses. One prominent example in this aspect is the human immunodeficiency virus-1 (HIV-1) [56]. As discussed earlier (Section 2.2), HIV infection facilitates the upregulation of DRs (as TNFR and Fas) and their ligands, which might also promote necroptosis sensitivity. A recent article demonstrates a HIV induced necrotic-like cell death in T cells, which can be inhibited by necrostatin-1, an inhibitor of RIPK1 [56] This in vitro work is supported by an in vivo study, in which RIPK3 knock down and necroptosis inhibition can restore the proliferation potential of CD8 T cells from HIV-infected patients [64].
3.2. The emerging pathway: ZBP-1 dependent necroptosis signalling in virus infection
The nucleic acid sensing receptor ZBP1, analogously to RIPKs, possess a RIPK homotypic interaction motif (RHIM), thus ZBP1 is capable to interact with RIPK3 [44]. RIPK3 then can initiate MLKL dependent necroptosis and can serve as a platform for caspase-8 dependent apoptosis (Fig. 2.) [45], thus only blocking of both pathways at the same time can significantly reduce IAV induced cell death. The importance of the ZBP1/RIPK3 axis as anti-viral defence is strongly supported by the studies on ZBP1 and RIPK3 deficient mice, which exhibit higher viral burden and are more susceptible to IAV induced lethality [45,65]. Importantly, it seems, if only one of the two cell death pathways triggered by ZBP1/RIPK3 functions, is already sufficient to ensure viral clearance, since only a simultaneous depletion of MLKL and caspase-8 function can lead to an increased IAV-driven increased lethality [45]. Taken together, apoptosis and necroptosis represent compensatory pathways upon IAV infection, yet necroptosis, as an immune-stimulatory cell death form renders a stronger adaptive immune stimulation [66]. Based on these studies, a limited activity of necroptosis can be considered as a beneficial measure to limit viral burden, however a drastic increase of cell death in the alveolar epithelial cells, which results in compromised lung function in mice and in humans, tightly correlates with lethality [67].
3.3. Viral anti-necroptotic proteins
To date, IAV infection represents the only viral infection which results in ZBP-1-driven necroptosis in its natural state, since all other necroptosis-inducing virus models employ genetically engineered viruses [64,65]. This well demonstrates that several viruses developed strategies to avoid necroptosis (Table 2.). Genetically engineered MCMV lacking the viral M45-encoded inhibitor of RIPK (vIRA) initiates premature cell death in mice and depletion of RIPK3 attenuates this cell death [68]. The MCMV induced necroptosis takes place independent of RIPK1 and TNF, yet it is triggered by ZBP1 in response to infection [69]. Conversely, both the wild type MCMV encoding vIRA and the human CMV [70] can efficiently evade RIPK3-dependent necroptosis initiation. The latent membrane protein-1 (LMP1) of Epstein-Barr virus (EBV) elicits the poly-ubiquitination of RIPKs and thereby inhibits necroptosis in nasopharyngeal epithelial cells [71]. Rhinoviruses, the causative agents of common cold express the viral 3C protease, which blocks Poly(I:C) stimulated necroptosis in airway epithelial cells by the direct cleavage of RIPK1 [72]. These virally encoded factors have one in common: they all target RIPKs in order to block necroptosis. A distinct inhibitory mechanism is provided by the Vaccinia virus (VACV) encoded innate immune evasion protein-3 (E3), which interacts with the putative RNA binding domain of ZBP1 and the genetic deletion of E3 leads to an immediate necroptosis in mouse L292 and human embryonal kidney cell lines [72]. Novel findings demonstrate a virally encoded MLKL homolog (vMLKL) in poxviruses, which exerts antagonistic effect in necroptosis initiation by binding to RIPK3 and thereby blocking the sequestration of human and mouse MLKL [73].
The species selectivity of viruses and the continuously ongoing evolutionary race between host and virus is well demonstrated by the following example of HSV-1. The viral ribonucleotide reductase ICP6 of HSV-1 possess a RHIM-like domain, thus it can engage both RIPK1 and RIPK3. Interestingly, HSV-1 infection leads to necroptosis in mice via ICP6 and is accompanied by limited propagation of the virus, whereas ICP-6 inhibits the necroptosis in humans, in the natural host of the virus. Furthermore, ICP10 the large subunit of the ribonucleotide reductase of HSV-2 can inhibit TNFR triggered necroptosis [74]. A recent study demonstrates that ICP6 blocks the TNFR stimulated MLKL-necrosome assembly into membrane vesicles [75]. This compartmentalization step represents a critical event towards necroptosis execution in humans, yet can be dispensable in mouse, which might answer why mouse cells are susceptible to HSV-1 triggered necroptosis.
4. Pyroptosis
4.1. The pyroptotic machinery
Pyroptosis is a regulated cell death form accompanied by osmotic imbalances and early membrane rupture of the cells [76]. Pyroptosis is conducted by inflammatory caspase activation and culminates in the release of pro-inflammatory cytokines. Upon stimulation, the inflammatory caspases, as caspase-1, caspase-4/5 in humans and caspase-11 in mice, are recruited in high molecular weight complexes termed inflammasomes [77]. Inflammasomes are considered as pathogen sensors that recognize PAMPs and based on the chemical nature of the PAMPs different inflammasomes are assembled. Inflammatory caspases, similar to initiator apoptotic caspases, possess a large pro-domain structure, which ensures the recruitment either directly or via an adaptor protein to PRRs. In turn, caspase-1 activation in the inflammasome leads to the processing of pro-interleukin-1β (IL-1β) and pro-IL-18, whereas activation of caspase-1, caspase-11 and caspase-4 can result in the cleavage of gasdermin-D (GSDMD), a member of a poorly studied protein family [78]. Consequently, the N-terminal region of GSDMD is inserted into the lipid bilayer of the cytoplasm membrane and forms oligomer pores [79] leading to release of mature ILs into the extracellular space (Fig. 3 .) [80]. The pore formation triggered osmotic imbalances and membrane ruptures can be interpreted as a collateral event, which takes place in only special circumstances and the primary event of IL release can, in fact, occur without cell destruction, yet not in every circumstances [81]. Indeed, Inflammasome-dependent pyroptosis is unleashed in response to various viral infections as follows: HIV, DV [82], IAV [83], Coxsacivirus-B3 (CVB3) [84], Human bocavirus 1 (HBoV1) [85], Hepatitis C virus [86] and Enterovirus71 (EV71) [87]. In some cases the presence of pyroptosis seems to be beneficial for the host by limiting the viral spread [88,89], however increasing number of studies show detrimental effect of pyroptosis in response to infections (Table 1.). Interestingly, unlike in the case of necroptosis and apoptosis, direct anti-pyroptotic measures of viruses are limited, which lets us to speculate that pyroptosis may be positioned later than apoptosis [90] and necroptosis in a putative virus-host co-evolutionary time-line. Intriguingly, one exemption is provided by EV71. The viral protease 3C of EV71 cleaves GSDMD into a non-functional form, which then unable to trigger pyroptosis in THP-1 macrophages and human embryonal kidney cells [90].
Fig. 3.
Pyroptosis signalling in virus infection.
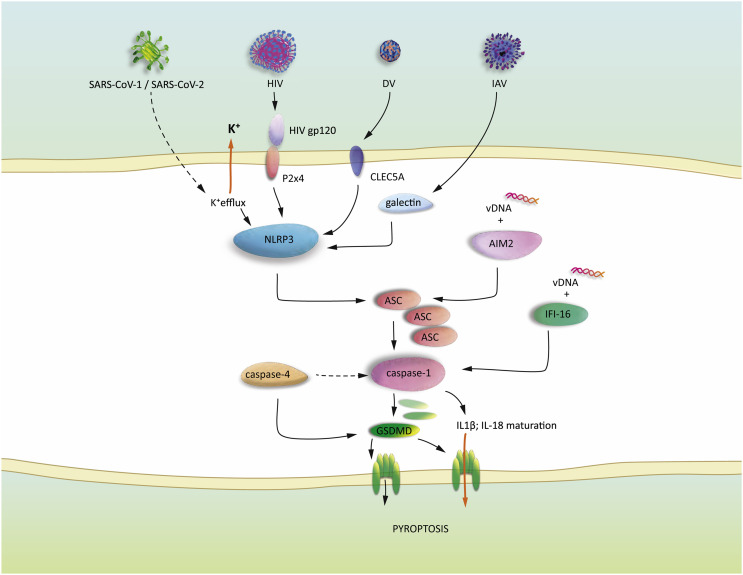
NLRP3 can be activated by various insults, including potassium efflux, extracellular ATP, and binding of virus associated patterns to host proteins (P2X4, CLEC5A, galectins). Activation of NLRP3 leads to the assembly of ASC specks, resulting in caspase-1 activation. vDNA is engaged by AIM2 resulting in ASC speck formation. Aberrant DNA fragments produced by HIV is recognized by IFI-16, which initiates caspase-1 activation. Active caspase-1 accomplishes the processing of GSDMD and pro-IL1β/ pro-IL18. Cleaved GSDMD oligomers form pores in the cytoplasm membrane. The mature ILs are released to the extracellular space throughout the GSDMD pores and the pore formation culminates in membrane ruptures and osmotic imbalances, ultimately leading to a lytic cell death, called pyroptosis.
4.2. Inflammasome activation in virus infection
The nucleotide binding oligomerization domain (NOD)-like receptors represent a broad group of inflammasome components, among which NOD-like receptor family pyrin domain containing 3 (NLRP3) is considered as the most studied one. In response to a danger signal, NLRP3 interacts the adaptor protein apoptosis-associated speck-like protein (ASC) via its pyrin domain thereby leading to ASC oligomerization and the formation of so called ASC specks. Importantly, ASC exhibits a caspase recruitment CARD domain, thus it is capable to recruit pro-inflammatory caspase-1 (Fig. 3) [91]. The conformational changes in NLRP3, which ultimately lead to activation, can be efficiently stimulated by uric acid crystals and extracellular ATP, however several studies conclude the unified role of osmotic imbalances and especially potassium efflux in activation [92]. Analogously, VSV and Encephalomyocarditis virus (EMCV) infections trigger NLRP3 inflammasome formation, which is initiated by a rapidly ongoing lytic cell death accompanied by potassium efflux [93]. Other studies provide some mechanistic clues, how NLRP3 is activated. For instance, IAV induces NLRP3 dependent pyroptosis in dendritic cells [83,94] and in respiratory epithelial cells [95]. The depletion of galectin-3, a β-galactosidase binding protein results in a strong inhibition of inflammation in lungs of the infected mice and co-immunoprecipitation experiments demonstrate the interaction of galectin-3, NLRP3 and ASC in bone marrow derived macrophages in response to avian IAV (H5N1) infection [96]. This study indicates that galectin-3 can serve as an alternative sensor in the NLRP3 inflammasome-driven pyroptosis. Along this line, gp120, an envelope protein of HIV induces inflammation and neuropathy in neurons. Interestingly, an ATP-dependent cationic ion channel purinergic 2 × 4 (P2X4) has been shown to initiate the gp120 triggered NLRP3 inflammasome assembly and caspase-1 activation [97]. DV has been also reported to stimulate NLRP3 inflammasome via the cytoplasmic membrane surface C-type lectin 5A (CLEC5A) [98]. Instead of stimulating NLRP3, DV can also directly stimulate caspase-4, the human homolog of capase-11 and leads to caspase-1 cleavage driven pyroptosis [99], thus, it seems , caspase-4 can function as an alternative PAMP sensor (Fig. 3.).
4.3. Inflammasome activation by viral nucleotide patterns
The recognition of viral nucleotide patterns play a crucial role in inflammasome induction. Activation of absent in melanoma-2 (AIM2) and interferon-γ-inducible protein 16 (IFI16) are two prominent examples for viral nucleotide pattern recognition (Fig. 3.).
AIM2 in the cytosol binds DNA fragments and recruits the adaptor ASC leading to ASC polymerization and assembly of the ASC specks. Interestingly, the upregulation of AIM2 has been observed in brain tissues after Zika virus infection, collected from fatal human cases [100]. This is therefore possible that the associated neuronal damage is linked to the over activation of AIM-2-dependent pyroptosis in neurons. Furthermore, EV71 [101], Vaccinia virus [102] and MCMV [103] infections have been shown to exert AIM2-driven pyroptosis. In contrast, HSV-1 inhibits AIM2 activation by the expression of VP22, a virally encoded factor, which interacts with the AIM2 protein and prevents the oligomerization of it. Conversely, Infection of HSV-1 lacking VP22 leads to reduced viral replication in vivo, which effect is restored in AIM2 deficient mice [104].
The DNA sensor interferon-γ-inducible protein 16 (IFI16) recognizes aberrant DNA transcripts, which are products of an incomplete virus replication cycle in HIV infected non-permissive CD4 T lymphocytes. The activation of IFI16 launches the caspase-1-driven pyroptotic pathway consequently attracting more immune cells to the site of infection and leading to an accelerated viral spread [105].
5. NETosis in response to virus infection
A neutrophil specific antimicrobial defence mechanism is represented by the release of neutrophil extracellular traps (NETs). NETs are released from the cells upon neutrophil cell death termed NETosis, and contain chromatin and antimicrobial proteins from the cytoplasm and granules of the cell [106]. The signalling pathways leading to NETosis are distinct from necroptosis [107] and apoptosis [108] and has been recently discussed in detail by Sollberger et al. [106]. Importantly, NETosis mostly occur in response to bacterial and fungal infections, yet some viruses, including HIV [109], Hanta virus [110] and Pox virus [111] can also trigger this specific cell death form exhibiting anti-viral properties. In contrast, a study on Human respiratory syncytial virus suggests that, although NETs trap viral particles, their exaggerated formation during severe cases plays a role in airway obstruction [112]. Furthermore, H5N1 influenza virus also triggers NETosis and this can similarly contribute to disease pathogenesis [113].
6. Cell death pathways in emerging coronaviruses
Severe acute respiratory syndrome (SARS) coronavirus (CoV, also: CoV-1) has led to a global outbreak of pneumonia resulting in approximately 800 deaths in 2003 [114]. Studies on human and mammalian cell lines demonstrate a SARS-CoV induced cytopathic effect, which exhibits caspase activation and typical apoptotic morphology [115]. Multiple viral proteins of SARS-CoV have been shown to contribute to apoptosis initiation, including the C terminal domain of SARS-CoV spike (S) protein [116], and the open reading frame (ORF) 7a and 3a proteins of the virus. The two ORF proteins employ distinct mechanisms to activate cell death, since SARS-CoV-ORF3a acts as a potassium channel and triggers caspase activation [117], whereas SARS-CoV-ORF7a directly interacts with BCL-XL [118], a pro-survival member of the BCL-2 family. It is, however, important to note that the above studies employ African green monkey kidney epithelial (Vero E6) cells, and not human cell lines. In contrast, if a human lung epithelial cell line is employed, then SARS-CoV-ORF3a binds RIPK3, which, however, does not lead to necroptotic MLKL activation, instead it facilitates a non-apoptotic lytic cell death [115].
Besides, SARS-CoV envelope (E) protein acts as an ion channel and is capable to activate NLRP3 in an in vitro model [119]. Interestingly, the lack of the ion channel activity of SARS-CoV-E does not influence virus replication in infected mice, yet reduces edema, the major factor of acute respiratory distress syndrome. The reduced edema correlates with preserved lung epithelia integrity and less IL-1β in the lung airways, which indicates a significant role for inflammasome activation in disease pathogenesis. [120]. The role of NLRP3 inflammasome assembly is further demonstrated in human macrophage cell lines, where the ORF8b protein of SARS-CoV directly binds NLRP3 and leads to pyroptotic cell death [121].
Middle East respiratory syndrome coronavirus (MERS-CoV) is a highly pathogenic beta-coronavirus strain with an approximately 30% case-fatality rate [122]. MERS-CoV infection of primary human T lymphocytes [123] and kidney and lung cells from human tissue [124] leads to apoptotic cell death, which might largely contribute to the high pathogenicity of the virus.
Novel studies on SARS-CoV-2, the pathogenic agent of the newly emerged coronavirus disease 2019 (COVID-19) already give some hints on the possible role of cell death in the infection caused tissue injury. Interestingly, the expression of ORF3a of SARS-CoV-2 is less cytotoxic than that of SARS-CoV-1, and stimulates less apoptosis and caspase activation in human cell lines [125]. This observation might explain why SARS-CoV-2 exhibits lower case-fatality rate than SARS-CoV-1 (~10%). Conversely, direct SARS-CoV-2 infection of organotypic airway epithelial cells results in higher cytopathic effect with apoptotic characteristic than infection with human coronavirus NL63, a less pathogenic member of the 7 known human coronaviruses [126].
Interesting novel data supports the evidence of NLRP3 inflammasome activation in response to SARS-CoV-2-S/Angiotensin-converting enzyme-2 receptor binding in hematopoietic and endothelial cells [127]. In this regard, the role of pyroptosis has not been investigated in detail yet, but one can speculate that the overactivation of NLRP3 inflammasome and the consequent pyroptotic cell death might be one of the contributing factors in severe cases of COVID-19. The ion channel property of SARS-CoV-2-E might represent an important factor in this context. This hypothesis will be very likely investigated in the near future.
7. Concluding remarks
Virus infection triggered cell death is a two edged sword, which might serve the purpose of the host by facilitating viral clearance in tissues where the increased cell destruction is not accompanied by function loss, yet in other cases it might assist viral propagation as the example of HIV infected CD4 T cells shows. The role of the three cell death modalities can be seen as redundant in the context of viral clearance, for instance in IAV infection, however they trigger immune stimulation at different extent. This information can be taken into consideration in designing novel therapies aiming at re-activating cell death pathways in dormant infected cells. Furthermore, increasing body of literature demonstrates the crosstalk between the discussed cell death pathways, thus manipulating one or the other cell death pathways may unavoidable influence the whole machinery. Along with this, the apoptotic effector caspase-6 [128] and the initiator caspase-8 can play a role in the necroptotic ZBP-1/RIPK3 axis, while, in other settings, caspase-8 may facilitate pyroptotic pathways [129]. These and other studies highlight that our knowledge of cell death pathways is still not complete, but it is growing continuously. The understanding of the fine tuning of these pathways might warrant better therapeutic options in treating viral diseases and preventing epidemics in the future.
Acknowledgements
The author is grateful to Dorothea Imre-Fecske and Emili Imre for the graphical design of the figures.
References
- 1.Doitsh G., Galloway N.L.K., Geng X., Yang Z., Monroe K.M., Zepeda O., Hunt P.W., Hatano H., Sowinski S., Muñoz-Arias I., Greene W.C. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow J., Franz K.M., Kagan J.C. PRRs are watching you: Localization of innate sensing and signaling regulators. Virology. 2015;479-480:104–109. doi: 10.1016/j.virol.2015.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerr J.F., Wyllie A.H., Currie A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fadok V.A., Voelker D.R., Campbell P.A., Cohen J.J., Bratton D.L., Henson P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992;7:2207–2216. [PubMed] [Google Scholar]
- 5.Cohen G.M. Caspases: The executioners of apoptosis. Biochem. J. 1997;326(Pt 1):1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mace P.D., Riedl S.J. Molecular cell death platforms and assemblies. Curr. Opin. Cell Biol. 2010;22:828–836. doi: 10.1016/j.ceb.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tait S.W.G., Green D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 8.Große L., Wurm C.A., Brüser C., Neumann D., Jans D.C., Jakobs S. Bax assembles into large ring-like structures remodeling the mitochondrial outer membrane in apoptosis. EMBO J. 2016;35:402–413. doi: 10.15252/embj.201592789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riedl S.J., Salvesen G.S. The apoptosome: Signalling platform of cell death. Nat. Rev. Mol. Cell Biol. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- 10.Wilson N.S., Dixit V., Ashkenazi A. Death receptor signal transducers: Nodes of coordination in immune signaling networks. Nat. Immunol. 2009;10:348–355. doi: 10.1038/ni.1714. [DOI] [PubMed] [Google Scholar]
- 11.Tewari M., Quan L.T., O'Rourke K., Desnoyers S., Zeng Z., Beidler D.R., Poirier G.G., Salvesen G.S., Dixit V.M. Yama/CPP32β, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 12.Halle S., Keyser K.A., Stahl F.R., Busche A., Marquardt A., Zheng X., Galla M., Heissmeyer V., Heller K., Boelter J., Wagner K., Bischoff Y., Martens R., Braun A., Werth K., Uvarovskii A., Kempf H., Meyer-Hermann M., Arens R., Kremer M., Sutter G., Messerle M., Förster R. In vivo killing capacity of cytotoxic T cells is limited and involves dynamic interactions and T cell cooperativity. Immunity. 2016;44:233–245. doi: 10.1016/j.immuni.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.S. Ariotti, J.B. Beltman, R. Borsje, M.E. Hoekstra, W.P. Halford, Haanen, John B A G, R.J. de Boer, T.N.M. Schumacher, subtle CXCR3-dependent chemotaxis of CTLs within infected tissue allows efficient target localization, J. Immunol. (Baltimore MD. 1950) 195 (2015) 5285–5295. 10.4049/jimmunol.1500853. [DOI] [PubMed]
- 14.Hickman H.D., Reynoso G.V., Ngudiankama B.F., Cush S.S., Gibbs J., Bennink J.R., Yewdell J.W. CXCR3 chemokine receptor enables local CD8(+) T cell migration for the destruction of virus-infected cells. Immunity. 2015;42:524–537. doi: 10.1016/j.immuni.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez J.A., Jenkins M.R., Rudd-Schmidt J.A., Brennan A.J., Danne J.C., Mannering S.I., Trapani J.A., Voskoboinik I. Rapid and unidirectional perforin pore delivery at the cytotoxic immune synapse. J. Immunol. (Baltimore MD. 1950) 2013;191:2328–2334. doi: 10.4049/jimmunol.1301205. [DOI] [PubMed] [Google Scholar]
- 16.Pinkoski M.J., Waterhouse N.J., Heibein J.A., Wolf B.B., Kuwana T., Goldstein J.C., Newmeyer D.D., Bleackley R.C., Green D.R. Granzyme B-mediated apoptosis proceeds predominantly through a Bcl-2-inhibitable mitochondrial pathway. J. Biol. Chem. 2001;276:12060–12067. doi: 10.1074/jbc.M009038200. [DOI] [PubMed] [Google Scholar]
- 17.Liao H., Xu J., Huang J. FasL/Fas pathway is involved in dengue virus induced apoptosis of the vascular endothelial cells. J. Med. Virol. 2010;82:1392–1399. doi: 10.1002/jmv.21815. [DOI] [PubMed] [Google Scholar]
- 18.Wang X., Tan J., Zoueva O., Zhao J., Ye Z., Hewlett I. Novel pandemic influenza A (H1N1) virus infection modulates apoptotic pathways that impact its replication in A549 cells. Microbes Infect. 2014;16:178–186. doi: 10.1016/j.micinf.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Kim K.-H., Seong B.L. Pro-apoptotic function of HBV X protein is mediated by interaction with c-FLIP and enhancement of death-inducing signal. EMBO J. 2003;22:2104–2116. doi: 10.1093/emboj/cdg210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paim A.C., Badley A.D., Cummins N.W. Mechanisms of human immunodeficiency virus-associated lymphocyte regulated cell death. AIDS Res. Hum. Retrovir. 2020;36:101–115. doi: 10.1089/AID.2019.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nie Z., Phenix B.N., Lum J.J., Alam A., Lynch D.H., Beckett B., Krammer P.H., Sekaly R.P., Badley A.D. HIV-1 protease processes procaspase 8 to cause mitochondrial release of cytochrome c, caspase cleavage and nuclear fragmentation. Cell Death Differ. 2002;9:1172–1184. doi: 10.1038/sj.cdd.4401094. [DOI] [PubMed] [Google Scholar]
- 22.Sainski A.M., Natesampillai S., Cummins N.W., Bren G.D., Taylor J., Saenz D.T., Poeschla E.M., Badley A.D. The HIV-1-specific protein Casp8p41 induces death of infected cells through Bax/Bak. J. Virol. 2011;85:7965–7975. doi: 10.1128/JVI.02515-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zevini A., Olagnier D., Hiscott J. Crosstalk between cytoplasmic RIG-I and STING sensing pathways. Trends Immunol. 2017;38:194–205. doi: 10.1016/j.it.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato H., Takeuchi O., Sato S., Yoneyama M., Yamamoto M., Matsui K., Uematsu S., Jung A., Kawai T., Ishii K.J., Yamaguchi O., Otsu K., Tsujimura T., Koh C.-S., Reis e Sousa C., Matsuura Y., Fujita T., Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 25.Yoneyama M., Fujita T. RIG-I family RNA helicases: Cytoplasmic sensor for antiviral innate immunity. Cytokine Growth Factor Rev. 2007;18:545–551. doi: 10.1016/j.cytogfr.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 26.Hou F., Sun L., Zheng H., Skaug B., Jiang Q.-X., Chen Z.J. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell. 2011;146:448–461. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seth R.B., Sun L., Ea C.-K., Chen Z.J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Belgnaoui S.M., Paz S., Hiscott J. Orchestrating the interferon antiviral response through the mitochondrial antiviral signaling (MAVS) adapter. Curr. Opin. Immunol. 2011;23:564–572. doi: 10.1016/j.coi.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Yap G.L.R., Sachaphibulkij K., Foo S.L., Cui J., Fairhurst A.-M., Lim L.H.K. Annexin-A1 promotes RIG-I-dependent signaling and apoptosis via regulation of the IRF3-IFNAR-STAT1-IFIT1 pathway in A549 lung epithelial cells. Cell Death Dis. 2020;11:463. doi: 10.1038/s41419-020-2625-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters K., Chattopadhyay S., Sen G.C. IRF-3 activation by Sendai virus infection is required for cellular apoptosis and avoidance of persistence. J. Virol. 2008;82:3500–3508. doi: 10.1128/JVI.02536-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Maadidi S., Faletti L., Berg B., Wenzl C., Wieland K., Chen Z.J., Maurer U., Borner C. A novel mitochondrial MAVS/Caspase-8 platform links RNA virus-induced innate antiviral signaling to Bax/Bak-independent apoptosis. J. Immunol. (Baltimore, MD. 1950) 2014;192:1171–1183. doi: 10.4049/jimmunol.1300842. [DOI] [PubMed] [Google Scholar]
- 32.Stawowczyk M., van Scoy S., Kumar K.P., Reich N.C. The interferon stimulated gene 54 promotes apoptosis. J. Biol. Chem. 2011;286:7257–7266. doi: 10.1074/jbc.M110.207068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eitz Ferrer P., Potthoff S., Kirschnek S., Gasteiger G., Kastenmüller W., Ludwig H., Paschen S.A., Villunger A., Sutter G., Drexler I., Häcker G. Induction of Noxa-mediated apoptosis by modified vaccinia virus Ankara depends on viral recognition by cytosolic helicases, leading to IRF-3/IFN-β-dependent induction of pro-apoptotic Noxa. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chattopadhyay S., Yamashita M., Zhang Y., Sen G.C. The IRF-3/Bax-mediated apoptotic pathway, activated by viral cytoplasmic RNA and DNA, inhibits virus replication. J. Virol. 2011;85:3708–3716. doi: 10.1128/JVI.02133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexopoulou L., Holt A.C., Medzhitov R., Flavell R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 36.Fitzgerald K.A., McWhirter S.M., Faia K.L., Rowe D.C., Latz E., Golenbock D.T., Coyle A.J., Liao S.-M., Maniatis T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 37.Weber A., Kirejczyk Z., Besch R., Potthoff S., Leverkus M., Häcker G. Proapoptotic signalling through Toll-like receptor-3 involves TRIF-dependent activation of caspase-8 and is under the control of inhibitor of apoptosis proteins in melanoma cells. Cell Death Differ. 2010;17:942–951. doi: 10.1038/cdd.2009.190. [DOI] [PubMed] [Google Scholar]
- 38.Feoktistova M., Geserick P., Kellert B., Dimitrova D.P., Langlais C., Hupe M., Cain K., MacFarlane M., Häcker G., Leverkus M. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishikawa H., Barber G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun L., Wu J., Du F., Chen X., Chen Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science (New York, N.Y.) 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diner B.A., Lum K.K., Toettcher J.E., Cristea I.M. Viral DNA sensors IFI16 and cyclic GMP-AMP synthase possess distinct functions in regulating viral gene expression, immune defenses, and apoptotic responses during herpesvirus infection. mBio. 2016;7 doi: 10.1128/mBio.01553-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White M.J., McArthur K., Metcalf D., Lane R.M., Cambier J.C., Herold M.J., van Delft M.F., Bedoui S., Lessene G., Ritchie M.E., Huang D.C.S., Kile B.T. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell. 2014;159:1549–1562. doi: 10.1016/j.cell.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ning X., Wang Y., Jing M., Sha M., Lv M., Gao P., Zhang R., Huang X., Feng J.-M., Jiang Z. Apoptotic caspases suppress type I interferon production via the cleavage of cGAS, MAVS, and IRF3. Mol. Cell. 2019;74:19–31. doi: 10.1016/j.molcel.2019.02.013. e7. [DOI] [PubMed] [Google Scholar]
- 44.Upton J.W., Kaiser W.J., Mocarski E.S. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11:290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nogusa S., Thapa R.J., Dillon C.P., Liedmann S., Oguin T.H., Ingram J.P., Rodriguez D.A., Kosoff R., Sharma S., Sturm O., Verbist K., Gough P.J., Bertin J., Hartmann B.M., Sealfon S.C., Kaiser W.J., Mocarski E.S., López C.B., Thomas P.G., Oberst A., Green D.R., Balachandran S. RIPK3 activates parallel pathways of MLKL-driven necroptosis and FADD-mediated apoptosis to protect against influenza a virus. Cell Host Microbe. 2016;20:13–24. doi: 10.1016/j.chom.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dürst M., Gissmann L., Ikenberg H., zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc. Natl. Acad. Sci. U. S. A. 1983;80:3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dufour F., Sasseville A.M.-J., Chabaud S., Massie B., Siegel R.M., Langelier Y. The ribonucleotide reductase R1 subunits of herpes simplex virus types 1 and 2 protect cells against TNFα- and FasL-induced apoptosis by interacting with caspase-8. Apoptosis Int. J. Program. Cell Death. 2011;16:256–271. doi: 10.1007/s10495-010-0560-2. [DOI] [PubMed] [Google Scholar]
- 48.Langelier Y., Bergeron S., Chabaud S., Lippens J., Guilbault C., Sasseville A.M.-J., Denis S., Mosser D.D., Massie B. The R1 subunit of herpes simplex virus ribonucleotide reductase protects cells against apoptosis at, or upstream of, caspase-8 activation. J. Gen. Virol. 2002;83:2779–2789. doi: 10.1099/0022-1317-83-11-2779. [DOI] [PubMed] [Google Scholar]
- 49.Thome M., Schneider P., Hofmann K., Fickenscher H., Meinl E., Neipel F., Mattmann C., Burns K., Bodmer J.L., Schröter M., Scaffidi C., Krammer P.H., Peter M.E., Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 50.Skaletskaya A., Bartle L.M., Chittenden T., McCormick A.L., Mocarski E.S., Goldmacher V.S. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc. Natl. Acad. Sci. U. S. A. 2001;98:7829–7834. doi: 10.1073/pnas.141108798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ebermann L., Ruzsics Z., Guzmán C.A., van Rooijen N., Casalegno-Garduño R., Koszinowski U., Čičin-Šain L. Block of death-receptor apoptosis protects mouse cytomegalovirus from macrophages and is a determinant of virulence in immunodeficient hosts. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Q., Snipas S., Orth K., Muzio M., Dixit V.M., Salvesen G.S. Target protease specificity of the viral serpin CrmA. Analysis of five caspases. J. Biol. Chem. 1997;272:7797–7800. doi: 10.1074/jbc.272.12.7797. [DOI] [PubMed] [Google Scholar]
- 53.Matsuoka M., Jeang K.-T. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat. Rev. Cancer. 2007;7:270–280. doi: 10.1038/nrc2111. [DOI] [PubMed] [Google Scholar]
- 54.Okamoto K., Fujisawa J.-i., Reth M., Yonehara S. Human T-cell leukemia virus type-I oncoprotein Tax inhibits Fas-mediated apoptosis by inducing cellular FLIP through activation of NF-kappaB. Genes Cells Devoted Mol. Cell. Mech. 2006;11:177–191. doi: 10.1111/j.1365-2443.2006.00927.x. [DOI] [PubMed] [Google Scholar]
- 55.Degterev A., Huang Z., Boyce M., Li Y., Jagtap P., Mizushima N., Cuny G.D., Mitchison T.J., Moskowitz M.A., Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 56.Pan T., Wu S., He X., Luo H., Zhang Y., Fan M., Geng G., Ruiz V.C., Zhang J., Mills L., Bai C., Zhang H. Necroptosis takes place in human immunodeficiency virus type-1 (HIV-1)-infected CD4+ T lymphocytes. PLoS One. 2014;9 doi: 10.1371/journal.pone.0093944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dondelinger Y., Delanghe T., Priem D., Wynosky-Dolfi M.A., Sorobetea D., Rojas-Rivera D., Giansanti P., Roelandt R., Gropengiesser J., Ruckdeschel K., Savvides S.N., Heck A.J.R., Vandenabeele P., Brodsky I.E., Bertrand M.J.M. Serine 25 phosphorylation inhibits RIPK1 kinase-dependent cell death in models of infection and inflammation. Nat. Commun. 2019;10:1729. doi: 10.1038/s41467-019-09690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McQuade T., Cho Y., Chan F.K.-M. Positive and negative phosphorylation regulates RIP1- and RIP3-induced programmed necrosis. Biochem. J. 2013;456:409–415. doi: 10.1042/BJ20130860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holler N., Zaru R., Micheau O., Thome M., Attinger A., Valitutti S., Bodmer J.L., Schneider P., Seed B., Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 60.Xie T., Peng W., Liu Y., Yan C., Maki J., Degterev A., Yuan J., Shi Y. Structural basis of RIP1 inhibition by necrostatins. Structure (London England 1993) 2013;21:493–499. doi: 10.1016/j.str.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 61.Tenev T., Bianchi K., Darding M., Broemer M., Langlais C., Wallberg F., Zachariou A., Lopez J., MacFarlane M., Cain K., Meier P. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol. Cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 62.O’Donnell M.A., Perez-Jimenez E., Oberst A., Ng A., Massoumi R., Xavier R., Green D.R., Ting A.T. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat. Cell Biol. 2011;13:1437–1442. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun L., Wang H., Wang Z., He S., Chen S., Liao D., Wang L., Yan J., Liu W., Lei X., Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 64.Gaiha G.D., McKim K.J., Woods M., Pertel T., Rohrbach J., Barteneva N., Chin C.R., Liu D., Soghoian D.Z., Cesa K., Wilton S., Waring M.T., Chicoine A., Doering T., Wherry E.J., Kaufmann D.E., Lichterfeld M., Brass A.L., Walker B.D. Dysfunctional HIV-specific CD8+ T cell proliferation is associated with increased caspase-8 activity and mediated by necroptosis. Immunity. 2014;41:1001–1012. doi: 10.1016/j.immuni.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thapa R.J., Ingram J.P., Ragan K.B., Nogusa S., Boyd D.F., Benitez A.A., Sridharan H., Kosoff R., Shubina M., Landsteiner V.J., Andrake M., Vogel P., Sigal L.J., tenOever B.R., Thomas P.G., Upton J.W., Balachandran S. DAI senses influenza A virus genomic RNA and activates RIPK3-dependent cell death. Cell Host Microbe. 2016;20:674–681. doi: 10.1016/j.chom.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yatim N., Jusforgues-Saklani H., Orozco S., Schulz O., Barreira da Silva R., Reis e Sousa C., Green D.R., Oberst A., Albert M.L. RIPK1 and NF-κB signaling in dying cells determines cross-priming of CD8+ T cells. Science (New York, N.Y.) 2015;350:328–334. doi: 10.1126/science.aad0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davidson S., Crotta S., McCabe T.M., Wack A. Pathogenic potential of interferon αβ in acute influenza infection. Nat. Commun. 2014;5:3864. doi: 10.1038/ncomms4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Upton J.W., Kaiser W.J., Mocarski E.S. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7:302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sridharan H., Ragan K.B., Guo H., Gilley R.P., Landsteiner V.J., Kaiser W.J., Upton J.W. Murine cytomegalovirus IE3-dependent transcription is required for DAI/ZBP1-mediated necroptosis. EMBO Rep. 2017;18:1429–1441. doi: 10.15252/embr.201743947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Omoto S., Guo H., Talekar G.R., Roback L., Kaiser W.J., Mocarski E.S. Suppression of RIP3-dependent necroptosis by human cytomegalovirus. J. Biol. Chem. 2015;290:11635–11648. doi: 10.1074/jbc.M115.646042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu X., Li Y., Peng S., Yu X., Li W., Shi F., Luo X., Tang M., Tan Z., Bode A.M., Cao Y. Epstein-Barr virus encoded latent membrane protein 1 suppresses necroptosis through targeting RIPK1/3 ubiquitination. Cell Death Dis. 2018;9:53. doi: 10.1038/s41419-017-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koehler H., Cotsmire S., Langland J., Kibler K.V., Kalman D., Upton J.W., Mocarski E.S., Jacobs B.L. Inhibition of DAI-dependent necroptosis by the Z-DNA binding domain of the vaccinia virus innate immune evasion protein, E3. Proc. Natl. Acad. Sci. U. S. A. 2017;114:11506–11511. doi: 10.1073/pnas.1700999114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petrie E.J., Sandow J.J., Lehmann W.I.L., Liang L.-Y., Coursier D., Young S.N., Kersten W.J.A., Fitzgibbon C., Samson A.L., Jacobsen A.V., Lowes K.N., Au A.E., Jousset Sabroux H., Lalaoui N., Webb A.I., Lessene G., Manning G., Lucet I.S., Murphy J.M. Viral MLKL homologs subvert necroptotic cell death by sequestering cellular RIPK3. Cell Rep. 2019;28:3309–3319. doi: 10.1016/j.celrep.2019.08.055. e5. [DOI] [PubMed] [Google Scholar]
- 74.Guo H., Omoto S., Harris P.A., Finger J.N., Bertin J., Gough P.J., Kaiser W.J., Mocarski E.S. Herpes simplex virus suppresses necroptosis in human cells. Cell Host Microbe. 2015;17:243–251. doi: 10.1016/j.chom.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ali M., Roback L., Mocarski E.S. Herpes simplex virus 1 ICP6 impedes TNF receptor 1-induced necrosome assembly during compartmentalization to detergent-resistant membrane vesicles. J. Biol. Chem. 2019;294:991–1004. doi: 10.1074/jbc.RA118.004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fink S.L., Cookson B.T. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell. Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 77.van Opdenbosch N., Lamkanfi M. Caspases in cell death, inflammation, and disease. Immunity. 2019;50:1352–1364. doi: 10.1016/j.immuni.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kayagaki N., Stowe I.B., Lee B.L., O'Rourke K., Anderson K., Warming S., Cuellar T., Haley B., Roose-Girma M., Phung Q.T., Liu P.S., Lill J.R., Li H., Wu J., Kummerfeld S., Zhang J., Lee W.P., Snipas S.J., Salvesen G.S., Morris L.X., Fitzgerald L., Zhang Y., Bertram E.M., Goodnow C.C., Dixit V.M. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 79.Sborgi L., Rühl S., Mulvihill E., Pipercevic J., Heilig R., Stahlberg H., Farady C.J., Müller D.J., Broz P., Hiller S. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35:1766–1778. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He W.-t., Wan H., Hu L., Chen P., Wang X., Huang Z., Yang Z.-H., Zhong C.-Q., Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Evavold C.L., Ruan J., Tan Y., Xia S., Wu H., Kagan J.C. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity. 2018;48:35–44. doi: 10.1016/j.immuni.2017.11.013. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan T.Y., Chu J.J.H. Dengue virus-infected human monocytes trigger late activation of caspase-1, which mediates pro-inflammatory IL-1β secretion and pyroptosis. J. Gen. Virol. 2013;94:2215–2220. doi: 10.1099/vir.0.055277-0. [DOI] [PubMed] [Google Scholar]
- 83.Kuriakose T., Man S.M., Malireddi R.K.S., Karki R., Kesavardhana S., Place D.E., Neale G., Vogel P., Kanneganti T.-D. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci. Immunol. 2016;1 doi: 10.1126/sciimmunol.aag2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y., Jia L., Shen J., Wang Y., Fu Z., Su S.-A., Cai Z., Wang J.-A., Xiang M. Cathepsin B aggravates coxsackievirus B3-induced myocarditis through activating the inflammasome and promoting pyroptosis. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1006872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deng X., Zou W., Xiong M., Wang Z., Engelhardt J.F., Ye S.Q., Yan Z., Qiu J. Human parvovirus infection of human airway epithelia induces pyroptotic cell death by inhibiting apoptosis. J. Virol. 2017;91 doi: 10.1128/JVI.01533-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kofahi H.M., Taylor N.G.A., Hirasawa K., Grant M.D., Russell R.S. Hepatitis C virus infection of cultured human hepatoma cells causes apoptosis and pyroptosis in both infected and bystander cells. Sci. Rep. 2016;6:37433. doi: 10.1038/srep37433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu X., Wu T., Chi Y., Ge Y., Wu B., Zhou M., Zhu F., Ji M., Cui L. Pyroptosis induced by enterovirus A71 infection in cultured human neuroblastoma cells. Virology. 2018;521:69–76. doi: 10.1016/j.virol.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 88.Aachoui Y., Leaf I.A., Hagar J.A., Fontana M.F., Campos C.G., Zak D.E., Tan M.H., Cotter P.A., Vance R.E., Aderem A., Miao E.A. Caspase-11 protects against bacteria that escape the vacuole. Science (New York, N.Y.) 2013;339:975–978. doi: 10.1126/science.1230751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kip E., Nazé F., Suin V., Vanden Berghe T., Francart A., Lamoral S., Vandenabeele P., Beyaert R., van Gucht S., Kalai M. Impact of caspase-1/11, -3, -7, or IL-1β/IL-18 deficiency on rabies virus-induced macrophage cell death and onset of disease. Cell Death Discov. 2017;3:17012. doi: 10.1038/cddiscovery.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lei X., Zhang Z., Xiao X., Qi J., He B., Wang J. Enterovirus 71 inhibits pyroptosis through cleavage of gasdermin D. J. Virol. 2017;91 doi: 10.1128/JVI.01069-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martinon F. Detection of immune danger signals by NALP3. J. Leukoc. Biol. 2008;83:507–511. doi: 10.1189/jlb.0607362. [DOI] [PubMed] [Google Scholar]
- 92.Martinon F., Pétrilli V., Mayor A., Tardivel A., Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 93.da Costa L.S., Outlioua A., Anginot A., Akarid K., Arnoult D. RNA viruses promote activation of the NLRP3 inflammasome through cytopathogenic effect-induced potassium efflux. Cell Death Dis. 2019;10:346. doi: 10.1038/s41419-019-1579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fernandez M.V., Miller E., Krammer F., Gopal R., Greenbaum B.D., Bhardwaj N. Ion efflux and influenza infection trigger NLRP3 inflammasome signaling in human dendritic cells. J. Leukoc. Biol. 2016;99:723–734. doi: 10.1189/jlb.3A0614-313RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee S., Hirohama M., Noguchi M., Nagata K., Kawaguchi A. Influenza A virus infection triggers pyroptosis and apoptosis of respiratory epithelial cells through the type I interferon signaling pathway in a mutually exclusive manner. J. Virol. 2018;92 doi: 10.1128/JVI.00396-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen Y.-J., Wang S.-F., Weng I.-C., Hong M.-H., Lo T.-H., Jan J.-T., Hsu L.-C., Chen H.-Y., Liu F.-T. Galectin-3 enhances avian H5N1 influenza a virus-induced pulmonary inflammation by promoting NLRP3 inflammasome activation. Am. J. Pathol. 2018;188:1031–1042. doi: 10.1016/j.ajpath.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 97.S. Zhao, Y. Zhou, Y. Fan, Y. Gong, J. Yang, R. Yang, L. Li, L. Zou, X. Xu, G. Li, S. Liu, C. Zhang, G. Li, S. Liang, Involvement of P2X4 receptor in gp120-induced pyroptosis in dorsal root ganglia, J. Neurochem. 10.1111/jnc.14850. [DOI] [PubMed]
- 98.Wu M.-F., Chen S.-T., Yang A.-H., Lin W.-W., Lin Y.-L., Chen N.-J., Tsai I.-S., Li L., Hsieh S.-L. CLEC5A is critical for dengue virus-induced inflammasome activation in human macrophages. Blood. 2013;121:95–106. doi: 10.1182/blood-2012-05-430090. [DOI] [PubMed] [Google Scholar]
- 99.Cheung K.T., Sze D.M.-Y., Chan K.H., Leung P.H.-M. Involvement of caspase-4 in IL-1 beta production and pyroptosis in human macrophages during dengue virus infection. Immunobiology. 2018;223:356–364. doi: 10.1016/j.imbio.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 100.de Sousa J.R., Azevedo R.d.S.d.S., Filho A.J. Martins, de Araujo M.T.F., Cruz E.d.R.M., Vasconcelos B.C.B., Cruz A.C.R., de Oliveira C.S., Martins L.C., Vasconcelos B.H.B., Casseb L.M.N., Chiang J.O., Quaresma J.A.S., Vasconcelos P.F.d.C. In situ inflammasome activation results in severe damage to the central nervous system in fatal Zika virus microcephaly cases. Cytokine. 2018;111:255–264. doi: 10.1016/j.cyto.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 101.Yogarajah T., Ong K.C., Perera D., Wong K.T. AIM2 inflammasome-mediated pyroptosis in enterovirus A71-infected neuronal cells restricts viral replication. Sci. Rep. 2017;7:5845. doi: 10.1038/s41598-017-05589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rathinam V.A.K., Jiang Z., Waggoner S.N., Sharma S., Cole L.E., Waggoner L., Vanaja S.K., Monks B.G., Ganesan S., Latz E., Hornung V., Vogel S.N., Szomolanyi-Tsuda E., Fitzgerald K.A. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hornung V., Ablasser A., Charrel-Dennis M., Bauernfeind F., Horvath G., Caffrey D.R., Latz E., Fitzgerald K.A. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maruzuru Y., Ichinohe T., Sato R., Miyake K., Okano T., Suzuki T., Koshiba T., Koyanagi N., Tsuda S., Watanabe M., Arii J., Kato A., Kawaguchi Y. Herpes simplex virus 1 VP22 inhibits AIM2-dependent inflammasome activation to enable efficient viral replication. Cell Host Microbe. 2018;23:254–265. doi: 10.1016/j.chom.2017.12.014. e7. [DOI] [PubMed] [Google Scholar]
- 105.Monroe K.M., Yang Z., Johnson J.R., Geng X., Doitsh G., Krogan N.J., Greene W.C. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science (New York, N.Y.) 2014;343:428–432. doi: 10.1126/science.1243640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sollberger G., Tilley D.O., Zychlinsky A. Neutrophil extracellular traps: The biology of chromatin externalization. Dev. Cell. 2018;44:542–553. doi: 10.1016/j.devcel.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 107.Kenny E.F., Herzig A., Krüger R., Muth A., Mondal S., Thompson P.R., Brinkmann V., von Bernuth H., Zychlinsky A. Diverse stimuli engage different neutrophil extracellular trap pathways. eLife. 2017;6 doi: 10.7554/eLife.24437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Remijsen Q., Vanden Berghe T., Wirawan E., Asselbergh B., Parthoens E., de Rycke R., Noppen S., Delforge M., Willems J., Vandenabeele P. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2011;21:290–304. doi: 10.1038/cr.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saitoh T., Komano J., Saitoh Y., Misawa T., Takahama M., Kozaki T., Uehata T., Iwasaki H., Omori H., Yamaoka S., Yamamoto N., Akira S. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. 2012;12:109–116. doi: 10.1016/j.chom.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 110.Raftery M.J., Lalwani P., Krautkrӓmer E., Peters T., Scharffetter-Kochanek K., Krüger R., Hofmann J., Seeger K., Krüger D.H., Schönrich G. β2 integrin mediates hantavirus-induced release of neutrophil extracellular traps. J. Exp. Med. 2014;211:1485–1497. doi: 10.1084/jem.20131092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jenne C.N., Wong C.H.Y., Zemp F.J., McDonald B., Rahman M.M., Forsyth P.A., McFadden G., Kubes P. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe. 2013;13:169–180. doi: 10.1016/j.chom.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 112.Cortjens B., de Boer O.J., de Jong R., Antonis A.F., Sabogal Piñeros Y.S., Lutter R., van Woensel J.B., Bem R.A. Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. J. Pathol. 2016;238:401–411. doi: 10.1002/path.4660. [DOI] [PubMed] [Google Scholar]
- 113.Chan L.L.Y., Nicholls J.M., Peiris J.S.M., Lau Y.L., Chan M.C.W., Chan R.W.Y. Host DNA released by NETosis in neutrophils exposed to seasonal H1N1 and highly pathogenic H5N1 influenza viruses. Respir. Res. 2020;21:160. doi: 10.1186/s12931-020-01425-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Drosten C., Günther S., Preiser W., van der Werf S., Brodt H.-R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A.M., Berger A., Burguière A.-M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.-C., Müller S., Rickerts V., Stürmer M., Vieth S., Klenk H.-D., Osterhaus A.D.M.E., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. New Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 115.Krähling V., Stein D.A., Spiegel M., Weber F., Mühlberger E. Severe acute respiratory syndrome coronavirus triggers apoptosis via protein kinase R but is resistant to its antiviral activity. J. Virol. 2009;83:2298–2309. doi: 10.1128/JVI.01245-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chow K.Y.C., Yeung Y.S., Hon C.C., Zeng F., Law K.M., Leung F.C.C. Adenovirus-mediated expression of the C-terminal domain of SARS-CoV spike protein is sufficient to induce apoptosis in Vero E6 cells. FEBS Lett. 2005;579:6699–6704. doi: 10.1016/j.febslet.2005.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chan C.-M., Tsoi H., Chan W.-M., Zhai S., Wong C.-O., Yao X., Chan W.-Y., Tsui S.K.-W., Chan H.Y.E. The ion channel activity of the SARS-coronavirus 3a protein is linked to its pro-apoptotic function. Int. J. Biochem. Cell Biol. 2009;41:2232–2239. doi: 10.1016/j.biocel.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tan Y.-X., Tan T.H.P., Lee M.J.-R., Tham P.-Y., Gunalan V., Druce J., Birch C., Catton M., Fu N.Y., Yu V.C., Tan Y.-J. Induction of apoptosis by the severe acute respiratory syndrome coronavirus 7a protein is dependent on its interaction with the Bcl-XL protein. J. Virol. 2007;81:6346–6355. doi: 10.1128/JVI.00090-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nieto-Torres J.L., Verdiá-Báguena C., Jimenez-Guardeño J.M., Regla-Nava J.A., Castaño-Rodriguez C., Fernandez-Delgado R., Torres J., Aguilella V.M., Enjuanes L. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology. 2015;485:330–339. doi: 10.1016/j.virol.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nieto-Torres J.L., DeDiego M.L., Verdiá-Báguena C., Jimenez-Guardeño J.M., Regla-Nava J.A., Fernandez-Delgado R., Castaño-Rodriguez C., Alcaraz A., Torres J., Aguilella V.M., Enjuanes L. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shi C.-S., Nabar N.R., Huang N.-N., Kehrl J.H. SARS-coronavirus open reading frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019;5:101. doi: 10.1038/s41420-019-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 123.Chu H., Zhou J., Wong B.H.-Y., Li C., Chan J.F.-W., Cheng Z.-S., Yang D., Wang D., Lee A.C.-Y., Li C., Yeung M.-L., Cai J.-P., Chan I.H.-Y., Ho W.-K., To K.K.-W., Zheng B.-J., Yao Y., Qin C., Yuen K.-Y. Middle east respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J. Infect. Dis. 2016;213:904–914. doi: 10.1093/infdis/jiv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yeung M.-L., Yao Y., Jia L., Chan J.F.W., Chan K.-H., Cheung K.-F., Chen H., Poon V.K.M., Tsang A.K.L., To K.K.W., Yiu M.-K., Teng J.L.L., Chu H., Zhou J., Zhang Q., Deng W., Lau S.K.P., Lau J.Y.N., Woo P.C.Y., Chan T.-M., Yung S., Zheng B.-J., Jin D.-Y., Mathieson P.W., Qin C., Yuen K.-Y. MERS coronavirus induces apoptosis in kidney and lung by upregulating Smad7 and FGF2. Nat. Microbiol. 2016;1:16004. doi: 10.1038/nmicrobiol.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ren Y., Shu T., Wu D., Mu J., Wang C., Huang M., Han Y., Zhang X.-Y., Zhou W., Qiu Y., Zhou X. The ORF3a protein of SARS-CoV-2 induces apoptosis in cells. Cell. Mol. Immunol. 2020;17:881–883. doi: 10.1038/s41423-020-0485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhu N., Wang W., Liu Z., Liang C., Wang W., Ye F., Huang B., Zhao L., Wang H., Zhou W., Deng Y., Mao L., Su C., Qiang G., Jiang T., Zhao J., Wu G., Song J., Tan W. Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells. Nat. Commun. 2020;11:3910. doi: 10.1038/s41467-020-17796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ratajczak M.Z., Bujko K., Ciechanowicz A., Sielatycka K., Cymer M., Marlicz W., Kucia M. SARS-CoV-2 entry receptor ACE2 is expressed on very small CD45(−) precursors of hematopoietic and endothelial cells and in response to virus spike protein activates the Nlrp3 inflammasome. Stem Cell Rev. Rep. 2020:1–12. doi: 10.1007/s12015-020-10010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zheng M., Karki R., Vogel P., Kanneganti T.-D. Caspase-6 Is a key regulator of innate immunity, inflammasome activation, and host defense. Cell. 2020;181:674–687. doi: 10.1016/j.cell.2020.03.040. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fritsch M., Günther S.D., Schwarzer R., Albert M.-C., Schorn F., Werthenbach J.P., Schiffmann L.M., Stair N., Stocks H., Seeger J.M., Lamkanfi M., Krönke M., Pasparakis M., Kashkar H. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature. 2019;575:683–687. doi: 10.1038/s41586-019-1770-6. [DOI] [PubMed] [Google Scholar]