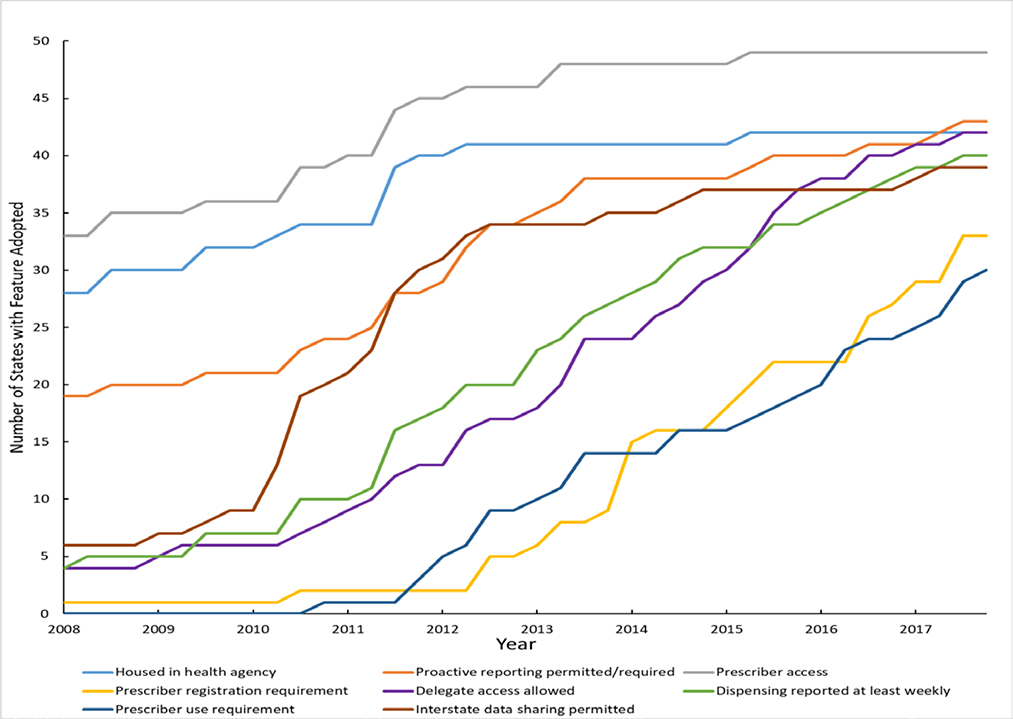
Figure. Prescription Drug Monitoring Program Feature Implementation, 2018–2017.
States with Selected Prescription Drug Monitoring Program Features, 2008–2017. *
*Data are from the author’s primary legal research and the Prescription Drug Abuse Policy System. “Housed in a health agency” means the agency in charge of PDMP operation is a state department of health, board of pharmacy, or professional licensing body. “Prescriber registration requirement” means the PDMP automatically enrolls prescribers or there is a requirement that prescribers enroll in (i.e., obtain login information for) the PDMP. “Prescriber use requirement” is a requirement that prescribers access PDMP data under specified clinical circumstances. “Proactive reporting permitted or required” means the PDMP operating agency is either required or permitted to proactively identify outlier prescribing, dispensing, or purchasing and report findings to professional licensing bodies, prescribers, dispensers, or law-enforcement officials. “Prescriber access” indicates prescribers can access PDMP data when registered with the system.