Abstract
Background:
Stress exacerbates symptoms of schizophrenia and attention deficit hyperactivity disorder, which are characterized by impairments in sustained attention. Yet how stress regulates attention remains largely unexplored. Here we investigated whether a 6-day variable stressor (VS) altered sustained attention and the cholinergic attention system in male and female rats.
Methods:
Sustained attention was tested with the sustained attention task (SAT). Successful performance on SAT relies on the release of acetylcholine (ACh) into the cortex from cholinergic neurons in the nucleus basalis of Meynert (NBM). Thus, we evaluated whether VS altered the morphology of these neurons with a novel approach using a Cre-dependent virus in genetically modified ChAT::Cre rats, a species used for this manipulation only. Next, electrochemical recordings measured cortical ACh following VS. Finally, we used RNAseq to identify VS-induced transcriptional changes in the NBM.
Results:
VS impaired attentional performance in SAT and increased the dendritic complexity of NBM cholinergic neurons in both sexes. NBM cholinergic neurons are mainly under inhibitory control, so this morphological change could increase inhibition on these neurons, reducing downstream ACh release to impair attention. Indeed, VS decreased ACh release in the prefrontal cortex of males. Quantification of global transcriptional changes revealed that, although VS induced many sex-specific changes in gene expression, it increased several signaling molecules in both sexes.
Conclusions:
These studies suggest that VS impairs attention by inducing molecular and morphological changes in the NBM. Identifying mechanisms by which stress regulates attention may guide the development of novel treatments for psychiatric disorders with attention deficits.
Keywords: sustained attention, basal forebrain, morphology, gene transcription, prefrontal cortex, sex as a biological variable
Introduction
Attentional impairments characterize many psychiatric disorders, including attention deficit hyperactivity disorder (ADHD) and schizophrenia(1, 2). Psychiatric disorders are also stress sensitive, with stress causing onset and exacerbating symptoms, including attentional deficits(3–5). Yet how stress modulates attention is poorly understood. Several studies have investigated effects of the stress neuropeptide, corticotropin releasing factor (CRF), on rodent attention tasks. Central administration of CRF impairs both selective and sustained attention in rats(6, 7). Additionally, maternal separation stress decreases the number of omitted trials in an attention task, but not other performance measures(8). Surprisingly, no studies have investigated whether repeated stressor exposure during adulthood impacts attention.
One way to study sustained attention in rodents is with the sustained attention task (SAT), which tests rats’ ability to monitor a situation for intermittent and unpredictable events(9). To succeed in the task, rats must distinguish between, and differentially respond to, signaled and non-signaled trials. An advantage of using SAT is that its underlying circuitry is fairly well-delineated and relies on the nucleus basalis of Meynert (NBM) in the basal forebrain(10). Lesions and optogenetic suppression of NBM cholinergic neurons disrupt performance on signaled trials, while optogenetic stimulation of these neurons enhances performance on signaled trials(11, 12). The phasic release of acetylcholine (ACh) into the medial prefrontal cortex (mPFC) is critical for signal detection(12, 13).
The present study aimed to determine how VS affected SAT and aspects of the underlying attention system in male and female rats. In other brain regions, stress can induce structural plasticity, so we assessed whether VS altered NBM cholinergic dendritic morphology. The effect of VS on ACh release in the mPFC was also evaluated. Finally, we quantified global transcriptional changes in the NBM following VS to identify molecular processes that could drive stress-induced changes in plasticity in the attention system.
Methods
Subjects
Procedures were approved by Temple University IACUC and consistent with NIH guidelines. Behavior and physiology studies used Sprague-Dawley rats (Charles River), given our history of studying stress and attention in this strain(7). Our technique to label NBM cholinergic neurons for morphology necessitated transgenic ChAT::Cre rats on a Long Evans background (Rat Resource and Research Center) that were genetically modified to express a restricted recombinase-driver (Cre) in the presence of the choline acetyltransferase (ChAT) promoter(14). To link morphology changes to transcriptional changes, wild type Long Evans (Charles River) rats were used. Importantly, both the Sprague-Dawley and Long Evans strains engage their cholinergic system for sustained attention and both strains respond to repeated stressors similarly(15–17). All animals were adults (70+ days), maintained in a 12-hour light/dark cycle (lights off at 8:30am), and pair-housed with access to food and water ad libitum, except where otherwise noted.
Variable stress (VS)
Day 1 of VS, rats were subjected to 1h of restraint in their home cage in a Broome restrainer. Day 2, rats were exposed for 15min to 100μl of 2,3,5-Trimethyl-3-thiazoline (TMT), a synthetic fox odor, which was pipetted onto a non-woven sponge and taped to the inside wall of an empty mouse cage. Day 3, rats were subjected to 15min of forced swim in a cylinder (40cm high, 18.5cm diameter) containing water 30cm deep, maintained at 23–25°C. Rats were dried off and placed under a heat lamp for 30min following swim stress. Days 4, 5, and 6 these three stressors repeated in the same order. Rats assigned to the control condition were left undisturbed in their home cages Days 1–6 (Fig.1A).
Figure 1. VS impaired SAT performance as assessed with the vigilance index.
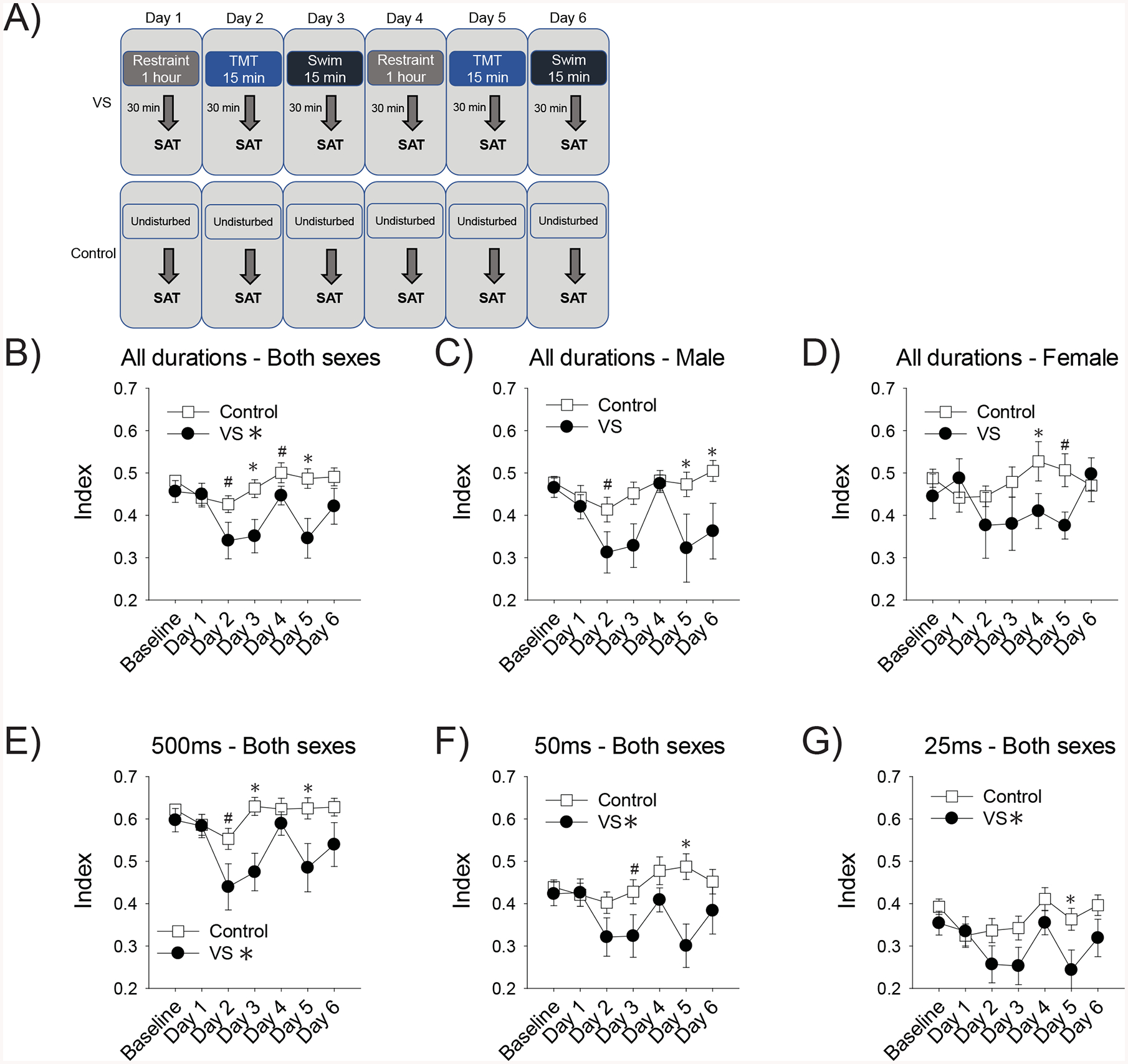
Timelines depict the VS and control manipulation and when these occurred relative to behavior testing (A). For the graphs, the first baseline measure on the x-axis reflects an average of 3 days of performance prior to exposure to VS or the control manipulation in Sprague-Dawley rats. Then daily performance 30 min after stressor cessation is depicted. VS impaired the vigilance index, an overall measure of attentional performance, in both sexes (B). Although there were no effects of sex, we presented the data from males (C) and females (D) separately on this measure for transparency with regards to sex as a biological variable. The vigilance index data was also analyzed separately for each of the three signaled-trial stimulus durations. VS impaired vigilance index performance at 500ms (E), 50ms (F), and 25ms (G). Asterisks indicate p< .05 from the control group, pound signs indicate a trend (p≤.10 and >.05)
SAT
Individually-housed adult male and female rats were food restricted to 85% of their free-feeding weight. Rats were trained in touchscreen SAT operant boxes as described in Supplementary Information (SI) and previously(18, 19). In brief, rats were trained to discriminate between signaled (durations 500ms, 50ms, or 25ms varied pseudorandomly) and non-signaled trials. Once they met criteria, VS groups (male n=13; female n=10) were then exposed to daily stressors and tested in SAT 30min after the cessation of each stressor. For the unstressed group (male n=16; female n=11), rats continued to run in SAT without any stressor exposure for 6 days after reaching criteria.
Attentional performance was assessed with the vigilance index, based on the proportion of hits and false alarms, such that a value of 0 indicated that the rat could not distinguish between signaled and non-signaled trials and a value of 1 indicated perfect performance (details in SI)(9).
Dendritic morphology
Rats underwent aseptic surgery as detailed in SI methods. The virus AAV9.CAG.Flex.eGFP.WPRE.bGH (from Dr. Hongkui Zeng of the Allen Institute for Brain Science via UPenn Viral Vector Core) was bilaterally infused at 1μL, 1×109gc/μL per side in the NBM as detailed (SI methods). Validation was done as described in SI with an anti-ChAT antibody and 92% of cells were positive for ChAT and the virus (SI Fig.1). Imaging analysis details are in SI methods. Group numbers are: unstressed male n=5 rats, n=26 cells; unstressed female n=4 rats, n=27 cells; male VS n=4 rats, n=22 cells; female VS n=4 rats, n=25 cells.
In vivo amperometric recordings
Electrodes were prepared as described in SI methods. All recordings were performed under anesthesia. Following the last manipulation, control (n=7 male, n=6 female) and VS (n= 6 male, n=6 female) rats were anesthetized with urethane (1.2–1.5g/kg,i.p), placed in the stereotax, and choline oxidase-coated microelectrodes were lowered into the right mPFC (AP: +3.0mm, ML: −0.7mm, DV: −2.7–3.0mm). A reference electrode was implanted in the rostral cortical region of the contralateral hemisphere. If microelectrodes failed to meet testing criterion during in vitro calibration, recording sessions were delayed. In these instances, rats were given additional stressor sessions for another 24–48h until a well-calibrated electrode was identified and recordings were completed. More details are in SI methods.
RNA extraction
A different cohort of rats was sacrificed by rapid decapitation 30min after the cessation of the final stressor in VS or the control procedure, mirroring the timing of the last behavioral test. The NBM was bilaterally dissected, frozen on dry ice, and stored at −80°C until RNA extraction (n=4/group) or qPCR validation (n=8/group). RNA was extracted as detailed in SI methods. RNA quality was assessed using Qubit RNA HS assay and BioanalyzerRNA6000 Nano assay. Libraries were prepared using NuGen Ovation RNA-Seq Systemv2 from total RNA and sequenced by UCLA Neuroscience Genomic Center (SI methods).
RNA-sequencing analysis and qPCR
Sequences were aligned to Rat Genome assembly (Rnor_6.0) using STAR-2.5.2a(20). rRNA reads were filtered using Bedtools intersectBed and rRNA annotation from Biomart. Gene read counts generated by HTseq-count were used to compute fold changes and significance of expression differences using DESeq(21). DEGs were assessed through a generalized linear model implemented in limma, with phenotype (VS vs. control) and sex (male vs. female) as main factors. The log expression values for each gene were averaged over treatment group, and the log2 fold change was computed. P-values were adjusted for multiple comparisons using Benjamin-Hochberg correction method. Heat maps of raw reads were generated using R’s pheatmap function. Unsupervised clustering was performed by pheatmap in default settings.
qPCR was performed on a separate cohort of animals to validate the three DEGs that were most significant with the highest fold change following stress found in males and females (see SI Methods). Fold change was calculated using 2−ΔΔCt analysis method.
Results
VS impairs SAT
Our main finding was that VS impaired attention in both sexes. A timeline depicting behavioral testing relative to the manipulations is shown in Figure 1A. Behavioral results were analyzed with mixed-factors ANOVAs. All analyses violated sphericity, so degrees of freedom were corrected with Greenhouse-Geisser estimates. Non-significant statistics reported in SI Results. A priori we predicted that differences in stress-induced attention deficits would not emerge until the later days of stress exposure (e.g., Days 4–6). Therefore, planned comparisons (using LSD posthocs) between control and VS rats were conducted for each day of behavior testing.
VS exposure reduced the vigilance index (signal durations combined), such that there was a main effect of condition in male and female rats [F(1,46)=6.95, p=.011, ] (Fig. 1B). Similarly, VS impaired attention (main effect) at each stimulus duration: 500ms [F(1,46)=8.56, p=.005, ], 50ms [F(1,46)=6.51, p=.014, ], and 25ms[F(1,46)=4.61 p=.037, ] (Fig.1E–G). There were no effects of sex, sex×condition, nor sex×condition×day interactions for any of the vigilance index measures. These findings indicate that stress impairs attentional performance regardless of sex. Figure 1.B–D shows the vigilance index combined data, as well as separated by sex for transparency regarding sex as a biological variable. Based on a priori predictions, we also analyzed data to assess the effect of stress at each individual day of the manipulation and trends (p≤.10 and >.05) and significant effects (p<.05) are indicated with pound signs and asterisks, respectively. Even though sex was not a significant factor in our analysis, these planned comparisons do suggest that TMT exposure on Day 5 disrupted performance in both sexes, while swim stress on Day 6 only affected males. These findings may indicate that either TMT is a more effective stressor for both sexes, or that females are quicker at recovering their attentional deficits after repeated stress.
Omissions were altered by VS and there was a day×condition interaction [F(3.16, 145.42)=5.73, p=.001, ] (SI Fig. 2). Full statistical analysis is reported in SI results. In short, omissions did not change over time in control rats, but changed in VS rats. Post-hoc tests revealed that omissions increased relative to baseline on days when rats were exposed to TMT and swim stress, but not restraint stress: days 2 (p=.037), 3 (p<.001), 6 (p=.005) with a trend for day 5 (p=.056). Increased omissions could indicate a disruption in attention or impaired motivation. Given that we did not see our overall measure of attentional performance, vigilance index, disrupted specifically by TMT and swim stress, it is more likely that these stressors decreased motivation to perform the task
VS alters NBM cholinergic dendritic morphology
VS induced dendritic hypertrophy in both sexes. Timeline for tissue collection (Fig. 2A). An image of a virally-labeled NBM cholinergic neuron in a ChAT::Cre rat (Fig. 2B). VS exposed ChAT::Cre rats had longer dendrites than control ChAT::Cre rats, as evidenced by a main effect of VS [F(1, 96)=4.88, p=.030, ], but no effect of sex nor interaction (Fig. 2C). A sex difference was found such that male dendrites had more nodes [F(1, 96)=18.60, p<.001, ] and ends [F(1, 96)=24.68,p<.001, ] than female dendrites (Fig. 2D,E). There were no main effects of VS or interactions for these measures.
Figure 2. VS increased the complexity of dendrites from NBM cholinergic neurons.
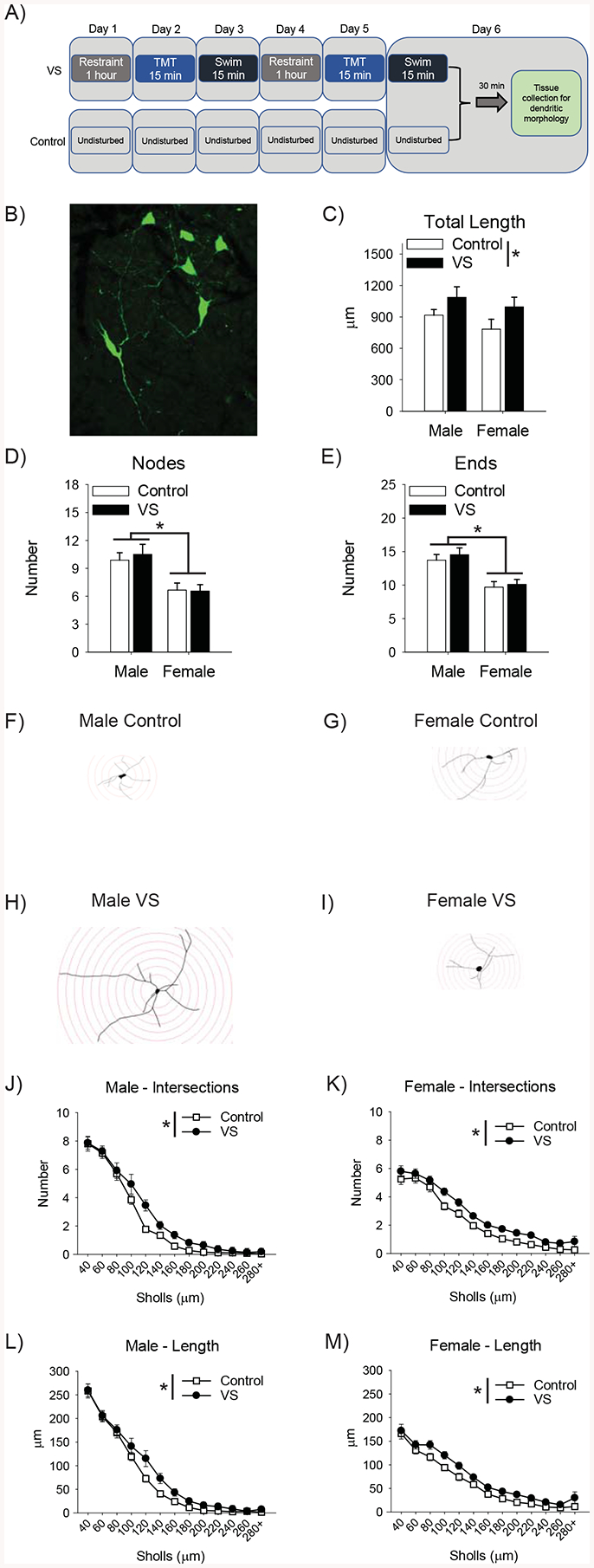
Timelines depict the VS and control manipulation and the timing of tissue collection (A). Image of a NBM cholinergic neuron labeled with a Cre-dependent virus in a ChAT::Cre rat (B). VS increased the total length of dendrites in both sexes (C). There was also a main effect of sex, such that male NBM cholinergic dendrites had more nodes and ends than those of females (D,E). Sholl analysis was performed to assess complexity as a function of distance from the cell body and a representative trace from each group is depicted (F–I). VS increased the number of intersections with the circles in both male (J) and female (K) rats. VS also increased the length within circles in both sexes (L,M).
Sholl analysis was conducted on intersections and dendritic length within the circles using a mixed factors ANOVA (sex×stress condition×distance) and revealed that VS induced dendritic hypertrophy. Representative Sholl analysis of neurons from each group (Fig. 2F–I). As distance from the cell body increased, intersections [F(12, 1152)=255.19, p<.001, ] and length [F(12,1152)=287.27, p< .001, ] decreased. There were sex×Sholl interactions for intersections [F(12, 1152)=12.15, p< .001, ] and length [F(12, 1152)=18.76, p< .001, ]. LSD post-hoc tests revealed female dendrites had fewer intersections (p<.05, 40–60μm) and were shorter near the cell body (p<.05, 40–80μm), but had more intersections (p<.05, 180–260μm) and were larger further away from the cell body (p<.05, 200–260μm). This analysis revealed a main effect of VS on intersections [F(1, 96)=4.18, p=.044, ] and length [F(1, 96)=4.78, p=.033, ], such that stress increased both measures (Fig. 2J–M). No other main effects or interactions were significant.
Electrochemical recording results
Timeline for typical electrochemical recordings (Fig. 3A). Examples of amperometric traces in response to depolarization for males (Fig. 3B) and females (Fig. 3C). There were baseline sex differences and sex-specific stress effects on depolarization-evoked ACh release in the mPFC of Sprague-Dawley rats as illustrated by VS×sex interaction [F(1,21)=6.37, p=.020, ]. LSD post-hoc tests revealed a baseline difference in evoked release such that control males released more ACh than control females (p=.023). There was a sex-specific effect of VS, such that VS decreased evoked release in males (p=.013), but not females (p=.385) relative to their unstressed same-sex counterparts (Fig. 3D).
Figure 3. VS reduced ACh release in the mPFC of males.
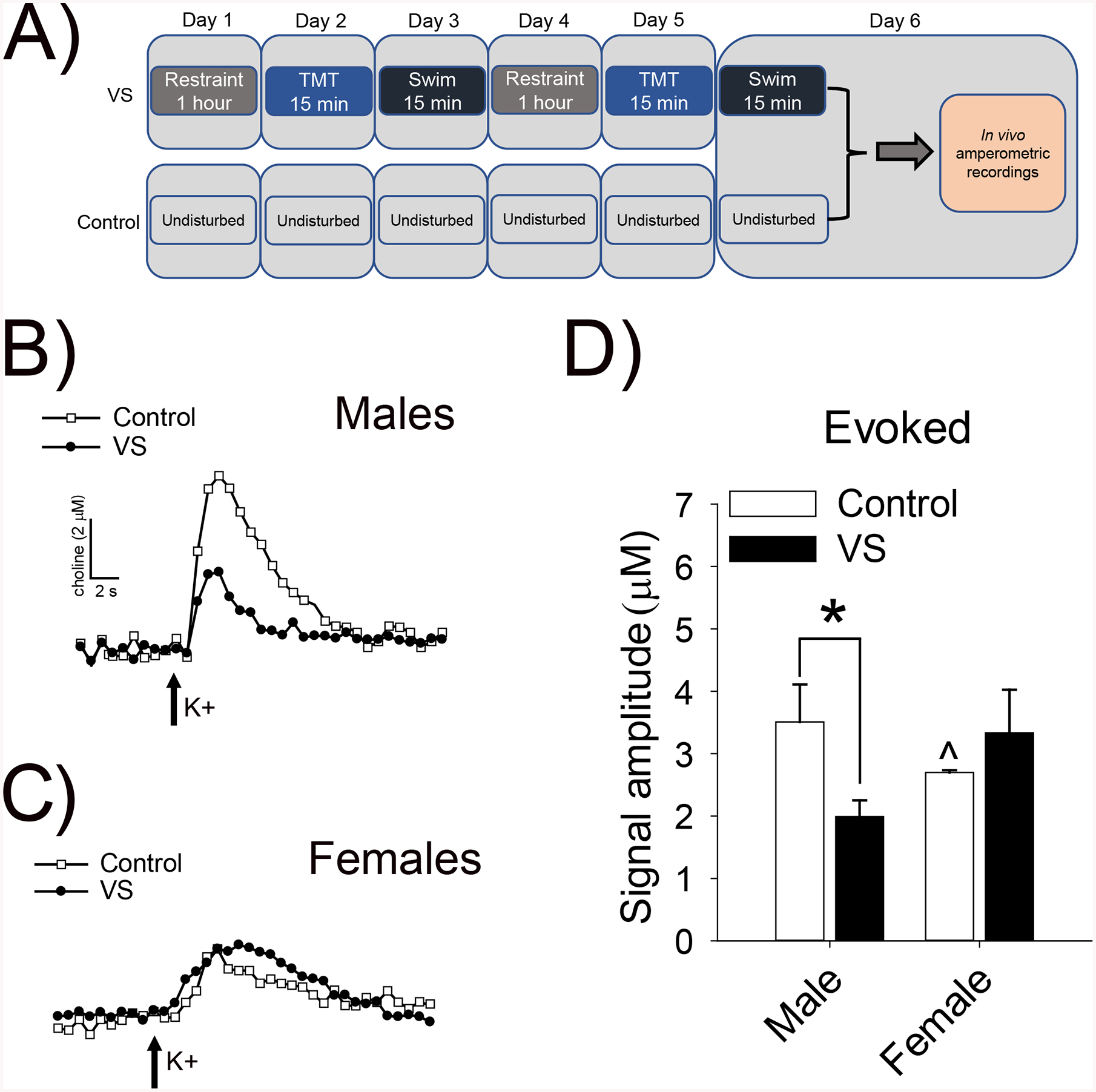
Timelines depict the typical timing for recordings following VS and control manipulations (A). Representative traces illustrating choline spikes following brief depolarizing pulses of potassium in the mPFC of control and VS exposed male (B) and female (C) Sprague-Dawley rats. Choline signals reflect the hydrolysis of newly-released ACh from presynaptic cholinergic terminals. VS reduced ACh release in male rats, but did not alter ACh release in female rats (D). Asterisk indicates p< .05 from the same sex control group and the caret indicates a sex difference between male and female control rats.
VS altered NBM gene transcription
Timeline for tissue collection (Fig. 4A). RNA-seq measured transcriptional changes in the NBM of control and VS rats. A heatmap of significantly regulated genes (p<.05) from the RNA-seq analysis (Fig. 4B,left). VS rats showed a distinct expression pattern compared to controls, (log2(FC)>1 or <−1; p<0.05). KEGG pathway analysis revealed VS regulated genes function in pathways such as Alanine, aspartate, and glutamate metabolism (Fig. 4B, right). KEGG pathway analyses were also conducted on genes altered by VS in males and females (i.e., sex specific) and many immune-related pathways were changed in both. Males had more pathways reach significance following VS than females (SI Table1). We also compared within sex, and heatmaps show significantly regulated genes for males and females (Fig. 4C–D). VS regulated more genes in female than in male rats (217 and 86, respectively) (Fig. 4E–F). Most genes were upregulated following VS in both females (73.7%) and males (63.95%). Across sexes, 14 genes were regulated in both male (16.28%) and female (6.45%) stressed animals (Fig. 4E–F). To validate our bioinformatic data, we selected the 3 most significantly VS-regulated genes in males (Dusp1, Pdk4, and Klf4) and the 3 most significantly VS-regulated genes in females (Dusp1, Dusp10, and Rfc5) and analyzed gene expression in a biological replicate cohort by qPCR in both sexes (Fig. 4G–K). qPCR confirmed differential expression of top genes, including the upregulation of Dual Specificity Phosphatase 1 (Dusp1) in males and females, which was the highest upregulated gene in both sexes identified with RNAseq. qPCR also revealed that Pdk4 and Klf4 were upregulated by VS only in males, while Dusp10 and Rfc5 were upregulated by VS only in females, and thus represent sex-specific gene changes.
Figure 4. VS alters gene transcription in the NBM.
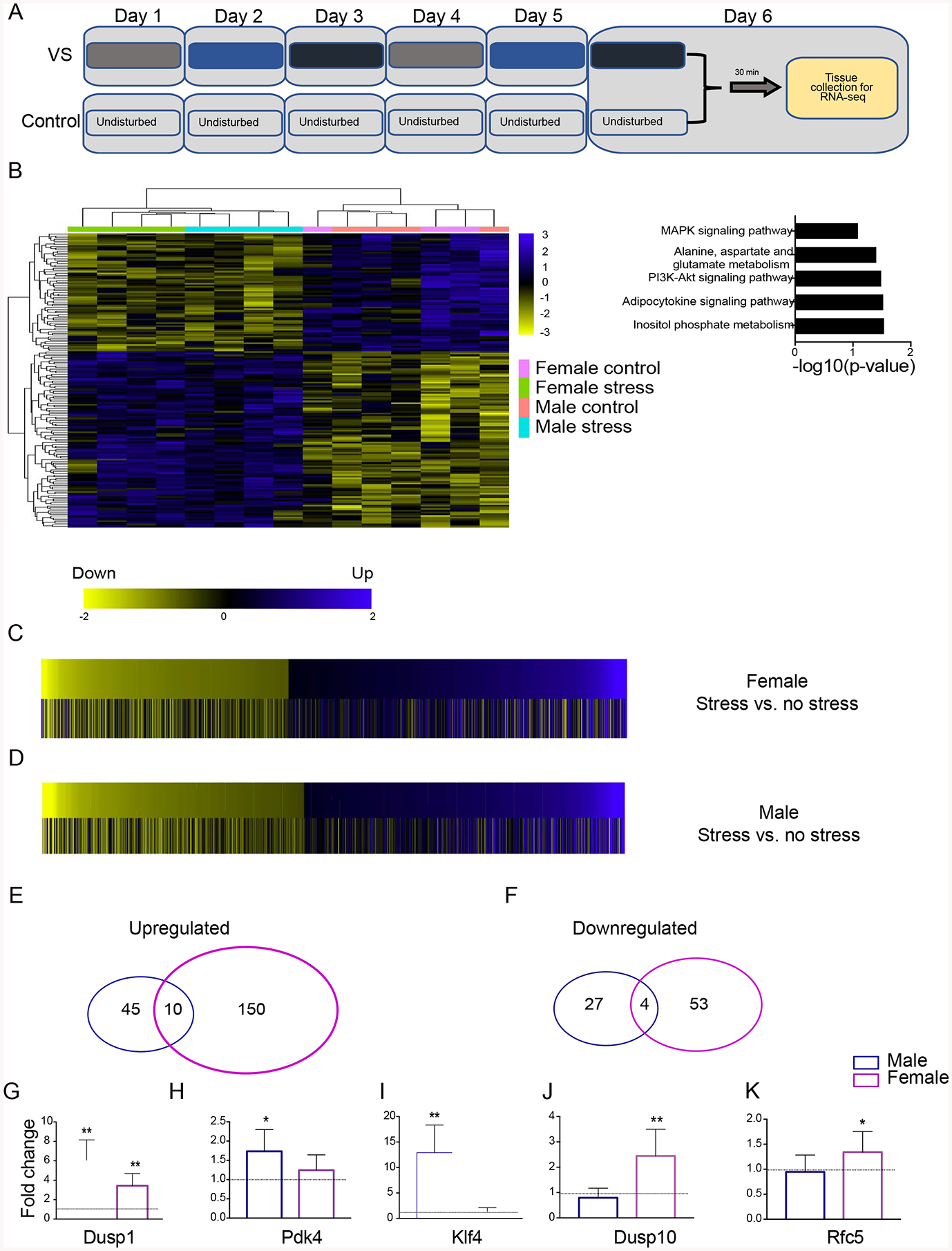
Timelines depict the timing for tissue collection following VS and control manipulations (A). Heatmap of genes differentially regulated by VS in Long Evans rats, with the counting reads of each gene plotted (B, left). KEGG pathway analysis on differentially expressed genes regulated by VS in both sexes (B, right). Heatmap of genes (with the log2FC plotted) differentially regulated by VS in females compared with the same genes in males (C), such that yellow bars indicate a negative log2FC (decreased gene expression by VS), black bars indicate no change by VS, and blue bars indicate a positive log2FC (increased gene expression by VS). Heatmap of genes (log2FC was plotted) differentially regulated by stress in males compared with females (D). Venn diagrams of total number of significantly upregulated genes (E) and downregulated genes (F) by VS in males (blue circle) and females (purple circle). Validation with qPCR in a different cohort of rats of the top 3 genes upregulated by VS in males: Dusp1 (G), Pdk4 (H), Klf4 (I); and females: Dusp1 (G), Dusp10 (J), Rfc5 (K). Notably, Dusp1 was the top gene upregulated in both sexes and the other genes were regulated by VS in a sex-specific manner.
Discussion
Human studies have associated life stress with attentional impairments(22–24), but previously the repeated effects of stress on sustained attention were not systematically assessed. Here we found that a 6-day VS procedure impaired the SAT vigilance index in male and female rats and induced dendritic hypertrophy in the NBM cholinergic neurons that underlie this task. These VS-induced changes were accompanied by a decrease in mPFC ACh release only in males, perhaps driven by an increase in inhibitory inputs to NBM cholinergic neurons as a result of the observed dendritic hypertrophy. VS exposure also caused sex-specific gene expression changes in NBM neurons, with more genes being up- and down-regulated in females than in males. However, in both sexes, the top upregulated gene following VS exposure was DUSP1. DUSP1 alters dendritic morphology in other regions(25), so changes in its expression may contribute to VS-induced dendritic hypertrophy.
Stress and attention
Here we addressed, for the first time, whether repeated stress in adulthood impairs sustained attention. Compared to controls, VS impaired attention in SAT in both male and female rats, although attention deficits in females appear to recover by the last stressor exposure. During SAT, the signal duration varies and VS impaired performance on all signal durations, indicating that stress was disruptive even when the signal was relatively easy to detect (500ms). One feature of our design was testing rats on SAT 30min after each daily stressor exposure, which makes it difficult to dissociate acute vs. chronic stress effects. There was no drop in performance following the first stressor exposure, so one possibility is that repeated stressor exposures are required to elicit attentional deficits. Another consideration is that different stressors vary in their impact on attention. Future studies are needed to dissociate the effects of repeated stress vs. stressor type on attention in males and females. Even with these limitations, the consequences of the disruptive effect of stress on sustained attention are likely impactful. Sustained attention subserves other forms of attention, including selective and divided attention, and is critical for higher order cognitive processes(10, 26). Therefore, cognitive deficits caused by stress may result, in part, from difficulty sustaining attention.
The stress response is complex and involves central and endocrine changes. The two modulators most associated with stress are: 1) glucocorticoids, which are released through HPA axis activation, and 2) CRF, which is released to initiate the HPA axis, as well as centrally to coordinate behavioral responses to stress. Human studies have not found a relationship between glucocorticoid levels and attentional processes(27–29). In contrast, central administration of CRF impairs SAT in male and female rats, and other cognitive processes that rely on sustained attention(6, 7, 30, 31). It is therefore likely that CRF plays a role in mediating the stress effects observed here, but future studies are needed to test this idea.
Stress induces dendritic hypertrophy in NBM cholinergic neurons
Accurate performance on SAT requires the release of ACh into the mPFC from NBM cholinergic neurons(12, 13). Therefore, stress could impair SAT by affecting NBM cholinergic neurons. Repeated stress impacts other neuron types by altering dendritic morphology(32–38). Existing tools to assess dendritic morphology in the basal forebrain have limitations: Golgi impregnation does not allow for the cell-type specificity; antibody staining does not fully label processes(39); and biocytin filling requires whole cell recording and only labels a small number of neurons that could be successfully patched(40). Thus, only a total of three basal forebrain cholinergic neurons from male rats had previously been reconstructed for morphology(40). In contrast, our approach of virally labeling cholinergic neurons in ChAT::Cre rats allows for cholinergic specificity, clear visualization of processes, and labeling of a large population of NBM cholinergic neurons. One caveat is that a recent publication found increased copies of the vesicular acetylcholine transporter gene in the prefrontal cortex of ChAT::Cre rats(41). It is not clear if this change would affect cholinergic dendritic morphology, but ChAT::Cre rats were used for both the control and VS conditions.
We first discovered a baseline sex difference in the shape of NBM cholinergic dendrites, with male dendrites being more complex than female dendrites. However, VS had the same effect on NBM cholinergic dendrites in both sexes, increasing their length and complexity. This stress-induced dendritic hypertrophy likely resulted from the repeated nature of the stressor, because it is typically chronic stress that is required to alter structural plasticity(32–38). Most prior studies exploring the effects of chronic stress on dendrites have focused on the hippocampus, amygdala, and PFC and there are examples of repeated stressors causing atrophy and hypertrophy depending on the region, manipulation, time after stressor cessation, and sex of the animal(32–38). Fewer studies have assessed the effects of stress on dendrites in ascending arousal systems. However, we previously found that lifelong overexpression of CRF increased the complexity of noradrenergic dendrites in the locus coeruleus (LC)-arousal system in male mice(42). This effect was not observed in females. However, female LC dendrites are longer and more complex than those of males at baseline, so perhaps stress could not induce further hypertrophy(42, 43). Given the widespread effects of ascending arousal circuits on cognition and sleep/wake, more studies investigating whether stress can alter the morphology of other cell groups are warranted.
Sex-specific effects of stress on the release of ACh into the mPFC
Changes in dendritic morphology affect how inputs are processed. Most NBM inputs, including parvalbumin, neuropeptide Y, and somatostatin neurons, inhibit cholinergic neurons (44–47). Given the high proportion of inhibitory inputs onto NBM cholinergic neurons, dendritic hypertrophy induced by VS could increase the inhibitory tone on these cells, ultimately reducing ACh output. We found that VS reduced ACh release in males, as predicted. Surprisingly, we did not observe a stress-induced change in ACh release in females, even though VS increased complexity of their NBM dendrites. The cause of this discrepancy is unclear. It is possible that stress alters inputs into cholinergic neurons differently in males and females. Perhaps in females, VS increases presynaptic excitatory synapses or reduces presynaptic inhibitory synapses on NBM cholinergic dendrites, negating an impact of dendritic hypertrophy on downstream ACh release.
The fact that stressed and unstressed females had similar levels of ACh release in the mPFC, yet VS impaired attention, raises questions about the cause of the attentional impairment in females. Here we focused on NBM cholinergic neurons, given that they are critical for attention, yet little is known about they are impacted by how stress. However, the mPFC and GABAergic neurons in the NBM also modulate certain aspects of SAT performance(10, 48, 49). Perhaps there are stress-induced changes in these other cell types that drive the female deficit in attention following VS.
The focus of this project was on the mechanisms by which VS can impair attention. However, in the process of studying the effects of stress we also discovered important baseline sex differences in the cholinergic attention system. These discoveries result from the fact that most previous studies typically used male rodents in their design, although McGaughy and Sarter (1999) demonstrated that, like in males, NBM cholinergic neurons were necessary for accurate SAT performance in female rats(50). Interestingly, male rats exhibited higher evoked ACh release following terminal depolarization under control conditions as compared to the female rats. Given the critical role of ACh release in sustained attention, one might predict that males would be better at SAT than females. Yet several studies, including this one, have found no sex differences in baseline SAT performance(7, 18). Females may instead have a downstream compensatory mechanism that allows them to adequately sustain attention, despite lower ACh levels. Females have more cortical nicotinic and muscarinic ACh receptors than males(51–53). Thus, the female mPFC may be better at detecting ACh release than the male mPFC, keeping female attention similar to male levels, despite lower ACh release at baseline.
Sex differences in gene transcription
To identify molecular processes by which stress could alter NBM neurons, we used RNAseq to assess VS-induced transcriptional changes in the NBM. Given that VS caused dendritic hypertrophy in both sexes, we first collapsed across sex to assess gene transcription. Many genes involved in signaling were upregulated by stress. Consistent with this finding, pathway analysis revealed changes in several signaling pathways including: inositol phosphate metabolism, which regulates calcium signaling; PI3K-Akt signaling; and mitogen-activated protein kinase (MAPK) signaling.
To assess whether top candidate genes altered by VS were similar in males and females, we also analyzed the sexes separately. VS caused a greater number of gene expression changes in females than in males, and this was true for both up- and down-regulated genes. Although VS induced dendritic hypertrophy in both sexes, only 14 genes were regulated in a similar direction in males and females. These data could indicate that there are sex-specific molecular processes underlying stress-induced dendritic hypertrophy of cholinergic neurons. Such sex-specificity exists in the mechanisms by which estrogens increase neuronal excitability in the hippocampus(54, 55).
Alternatively, our analysis indicates that some genes are similarly altered by VS in both sexes, so these genes may be the ones critical for morphological changes. The top gene upregulated by stress for both sexes was DUSP1, also known as mitogen-activated protein kinase phosphatase-1. MAPKs, including p38, ERK and JNK, are signal transducing enzymes that influence proliferation, differentiation, development, transformation, and apoptosis. DUSPs dephosphorylate MAPKs at threonine and tyrosine residues, inactivating their function(56, 57). DUSP1 is a stress-inducible protein that can regulate dendritic morphology(25, 58–60). Specifically, DUSP1 overexpression in developing dopaminergic neurons increases dendritic branching and length(25). Our results suggest that the VS-induced expression of DUSP1 could promote cholinergic dendritic hypertrophy. Given that tissue collection occurred 30min after the final stressor cessation, it remains unclear whether transcriptional changes in the NBM result from acute vs. chronic stressor exposure. There is evidence that chronic stress increases DUSP1 in the ventrolateral orbital cortex and hippocampus(59, 60). Moreover, typically chronic stress causes changes in dendritic morphology and we believe that many of these transcriptional changes are linked to the observed changes in structural plasticity(32–38). It is therefore likely it is the chronic nature of the VS manipulation that increase DUSP1 to promote dendritic hypertrophy. However, future studies are required to test this idea.
Conclusion
Stress impairs sustained attention in male and female rats. It also alters the morphology of cholinergic neurons in the attention circuit and induces sex-specific changes in cortical ACh release. Given that sustained attention underlies higher order cognitive processes and is disrupted in several psychiatric disorders, understanding the molecular processes by which stress impairs this attention system may lead to novel treatments to improve cognitive function.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | Cat # MAB305 | |||
| Bacterial or Viral Strain | University of Pennsylvania, Viral Vector Core | N/A | ||
| Biological Sample | ||||
| Cell Line | ||||
| Chemical Compound or Drug | ||||
| Commercial Assay Or Kit | ||||
| Deposited Data; Public Database | ||||
| Genetic Reagent | ||||
| Organism/Strain | rat: Long Evans-Tg(ChAT-Cre)5.1Deis, male and female | Rat Resource and Research Center | ||
| Organism/Strain | rat: Sprague Dawley, male and female | Charles River | ||
| Organism/Strain | rat: Long-Evans, male and female | Charles River | RRID:RGD_2308852 | |
| Peptide, Recombinant Protein | Choline oxidase from Alcaligenes Sp. (E.C.1.1.3.17) | Millipore Sigma | Cat # C5896 | |
| Recombinant DNA | ||||
| Sequence-Based Reagent | ||||
| Software; Algorithm | Neurolucida | MBF Bioscience Inc. | RRID:SCR_001775 | |
| Software; Algorithm | FAST-16 data acquisition | Quanteon | N/A | |
| Transfected Construct | ||||
| Commercial Assay Or Kit | iScript cDNA Synthesis Kit | Bio-Rad | 1708891BUN | |
| Commercial Assay Or Kit | RNeasy Mini Kit | Qiagen | 74106 | |
| Commercial Assay Or Kit | Universal RNA-Seq Library Preparation Kit | TECAN | Part No. 7102 | |
| Sequence-Based Reagent | Primers for RT-qPCR, see Supplement methods | This paper | N/A | |
| Software; Algorithm | STAR | PMID: 23104886 | STAR/2.5.2a | |
| Software; Algorithm | HTSeq Python package | PMID: 25260700 | HTseq-count | |
| Software; Algorithm | DESeq | PMID: 20979621 | ||
| Deposited data | GSE147806 | NCBI GEO DataSets | https://www.ncbi.nlm.nih.gov/gds | |
| Software; Algorithm | samtools | PMID: 19505943 | samtools-1.1 | |
| Software; Algorithm | bedtools | PMID: 25199790 | ||
| Other |
Acknowledgments
An earlier version of the behavioral, molecular, and electrochemical data was published in an abstract in Neuropsychopharmacology for the 2018 American College of Neuropsychopharmacology meeting. We would like to thank Natalie Newcamp, Evelyn Ordoñes Sanchez, Kimberly Wiersielis, and Jessica Tucci for their technical assistance. This work was supported by T32 DA007237 (SRE), NIH K99/R00 MH092438 (DAB), NSF CAREER grant IOS-1552416 (DAB), NSF grant IOS- 1929829 (DAB), Pennsylvania Department of Health grant 420792 (DAB), iGEM Award for Genomics from Temple University (DAB), Charles E Kaufman Foundation Young Investigator Award (EAH), NIH-NIDA Avenir Director’s Pioneer Award DP1 DA044250 (EAH), Brain and Behavior Research Foundation 570769 (EAH,) R00 MH090237 (MRA), and NIH AG046580 (VP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None of the authors have biomedical financial interests or potential conflicts of interest.
References
- 1.Magnin E, Maurs C (2017): Attention-deficit/hyperactivity disorder during adulthood. Revue Neurologique. 173:506–515. [DOI] [PubMed] [Google Scholar]
- 2.Hoonakker M, Doignon-Camus N, Bonnefond A (2017): Sustaining attention to simple visual tasks: a central deficit in schizophrenia? A systematic review. Annals of the New York Academy of Sciences. 1408:32–45. [DOI] [PubMed] [Google Scholar]
- 3.Newman SC, Bland RC (1994): Life events and the 1-year prevalence of major depressive episode, generalized anxiety disorder, and panic disorder in a community sample. Comprehensive Psychiatry. 35:76–82. [DOI] [PubMed] [Google Scholar]
- 4.Hirvikoski T, Lindholm T, Nordenström A, Nordström A-L, Lajic S (2009): High self-perceived stress and many stressors, but normal diurnal cortisol rhythm, in adults with ADHD (attention-deficit/hyperactivity disorder). Hormones and Behavior. 55:418–424. [DOI] [PubMed] [Google Scholar]
- 5.Holtzman CW, Trotman HD, Goulding SM, Ryan AT, MacDonald AN, Shapiro DI, et al. (2013): Stress and neurodevelopmental processes in the emergence of psychosis. Neuroscience. 249:172–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van’t Veer A, Yano JM, Carroll FI, Cohen BM, Carlezon WA Jr. (2012): Corticotropin-releasing factor (CRF)-induced disruption of attention in rats is blocked by the kappa-opioid receptor antagonist JDTic. Neuropsychopharmacology. 37:2809–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole RD, Kawasumi Y, Parikh V, Bangasser DA (2016): Corticotropin releasing factor impairs sustained attention in male and female rats. Behav Brain Res. 296:30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boutros N, Der-Avakian A, Markou A, Semenova S (2017): Effects of early life stress and adolescent ethanol exposure on adult cognitive performance in the 5-choice serial reaction time task in Wistar male rats. Psychopharmacology. 234:1549–1556. [DOI] [PubMed] [Google Scholar]
- 9.McGaughy J, Sarter M (1995): Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology (Berl). 117:340–357. [DOI] [PubMed] [Google Scholar]
- 10.Sarter M, Givens B, Bruno JP (2001): The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain research Brain research reviews. 35:146–160. [DOI] [PubMed] [Google Scholar]
- 11.McGaughy J, Kaiser T, Sarter M (1996): Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behavioral neuroscience. 110:247–265. [DOI] [PubMed] [Google Scholar]
- 12.Gritton HJ, Howe WM, Mallory CS, Hetrick VL, Berke JD, Sarter M (2016): Cortical cholinergic signaling controls the detection of cues. Proceedings of the National Academy of Sciences. 113:E1089–E1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parikh V, Kozak R, Martinez V, Sarter M (2007): Prefrontal Acetylcholine Release Controls Cue Detection on Multiple Timescales. Neuron. 56:141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, et al. (2011): Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 72:721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konkle ATM, Baker SL, Kentner AC, Barbagallo LS-M, Merali Z, Bielajew C (2003): Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Research. 992:227–238. [DOI] [PubMed] [Google Scholar]
- 16.Botly LCP, De Rosa E (2011): Impaired Visual Search in Rats Reveals Cholinergic Contributions to Feature Binding in Visuospatial Attention. Cerebral Cortex. 22:2441–2453. [DOI] [PubMed] [Google Scholar]
- 17.Kucinski A, Phillips KB, Koshy Cherian A, Sarter M (2020): Rescuing the attentional performance of rats with cholinergic losses by the M1 positive allosteric modulator TAK-071. Psychopharmacology. 237:137–153. [DOI] [PubMed] [Google Scholar]
- 18.Bangasser DA, Wicks B, Waxler DE, Eck SR (2017): Touchscreen Sustained Attention Task (SAT) for Rats. Journal of Visualized Experiments. 127:e56219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wicks B, Waxler DE, White KM, Duncan N, Bergmann J, Cole RD, et al. (2017): Method for testing sustained attention in touchscreen operant chambers in rats. Journal of Neuroscience Methods. 277:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. (2012): STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 29:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anders S, Huber W (2010): Differential expression analysis for sequence count data. Genome Biology. 11:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hancock PA, Warm JS (1989): A dynamic model of stress and sustained attention. Hum Factors. 31:519–537. [DOI] [PubMed] [Google Scholar]
- 23.Linden DVD, Keijsers GPJ, Eling P, Schaijk RV (2005): Work stress and attentional difficulties: An initial study on burnout and cognitive failures. Work & Stress. 19:23–36. [Google Scholar]
- 24.Szalma JL (2009): Individual differences in performance, workload, and stress in sustained attention: Optimism and pessimism. Personality and Individual Differences. 47:444–451. [Google Scholar]
- 25.Collins LM, O’Keeffe GW, Long-Smith CM, Wyatt SL, Sullivan AM, Toulouse A, et al. (2013): Mitogen-Activated Protein Kinase Phosphatase (MKP)-1 as a Neuroprotective Agent: Promotion of the Morphological Development of Midbrain Dopaminergic Neurons. NeuroMolecular Medicine. 15:435–446. [DOI] [PubMed] [Google Scholar]
- 26.Smilek D, Carriere JSA, Cheyne JA (2010): Failures of sustained attention in life, lab, and brain: Ecological validity of the SART. Neuropsychologia. 48:2564–2570. [DOI] [PubMed] [Google Scholar]
- 27.Newcomer JW, Selke G, Melson AK, et al. (1999): DEcreased memory performance in healthy humans induced by stress-level cortisol treatment. Archives of general psychiatry. 56:527–533. [DOI] [PubMed] [Google Scholar]
- 28.Lupien SJ, Gillin CJ, Hauger RL (1999): Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: A dose–response study in humans. Behavioral neuroscience. 113:420. [DOI] [PubMed] [Google Scholar]
- 29.Newcomer JW, Craft S, Hershey T, Askins K, Bardgett ME (1994): Glucocorticoid-induced impairment in declarative memory performance in adult humans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 14:2047–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hupalo S, Berridge CW (2016): Working Memory Impairing Actions of Corticotropin-Releasing Factor (CRF) Neurotransmission in the Prefrontal Cortex. Neuropsychopharmacology. 41:2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bryce CA, Floresco SB (2016): Perturbations in Effort-Related Decision-Making Driven by Acute Stress and Corticotropin-Releasing Factor. Neuropsychopharmacology. 41:2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS (2012): Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 37:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grillo CA, Risher M, Macht VA, Bumgardner AL, Hang A, Gabriel C, et al. (2015): Repeated restraint stress-induced atrophy of glutamatergic pyramidal neurons and decreases in glutamatergic efflux in the rat amygdala are prevented by the antidepressant agomelatine. Neuroscience. 284:430–443. [DOI] [PubMed] [Google Scholar]
- 34.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S (2002): Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 22:6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S (2005): Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 102:9371–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH (2010): Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex. 20:2560–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garrett JE, Wellman CL (2009): Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience. 162:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS (1997): Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 81:689–697. [DOI] [PubMed] [Google Scholar]
- 39.Dinopoulos A, Parnavelas JG, Eckenstein F (1986): Morphological characterization of cholinergic neurons in the horizontal limb of the diagonal band of Broca in the basal forebrain of the rat. Journal of Neurocytology. 15:619–628. [DOI] [PubMed] [Google Scholar]
- 40.Duque A, Tepper JM, Detari L, Ascoli GA, Zaborszky L (2007): Morphological characterization of electrophysiologically and immunohistochemically identified basal forebrain cholinergic and neuropeptide Y-containing neurons. Brain Structure and Function. 212:55–73. [DOI] [PubMed] [Google Scholar]
- 41.Mantanona CP, Alsiö J, Elson JL, Fisher BM, Dalley JW, Bussey T, et al. (2019): Altered motor, anxiety-related and attentional task performance at baseline associate with multiple gene copies of the vesicular acetylcholine transporter and related protein overexpression in ChAT::Cre+ rats. Brain Structure and Function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bangasser DA, Reyes BA, Piel D, Garachh V, Zhang XY, Plona ZM, et al. (2013): Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression. Mol Psychiatry. 18:166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bangasser DA, Zhang X, Garachh V, Hanhauser E, Valentino RJ (2011): Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal. Physiol Behav. 103:342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu M, Chung S, Zhang S, Zhong P, Ma C, Chang W-C, et al. (2015): Basal forebrain circuit for sleep-wake control. Nature Neuroscience. 18:1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaborszky L, van den Pol A, Gyengesi E (2012): The basal forebrain cholinergic projection system in mice. The mouse nervous system.684–714. [Google Scholar]
- 46.Zaborszky L, Gaykema RP, Swanson DJ, Cullinan WE (1997): Cortical input to the basal forebrain. Neuroscience. 79:1051–1078. [DOI] [PubMed] [Google Scholar]
- 47.Duque A, Balatoni B, Detari L, Zaborszky L (2000): EEG Correlation of the Discharge Properties of Identified Neurons in the Basal Forebrain. Journal of Neurophysiology. 84:1627–1635. [DOI] [PubMed] [Google Scholar]
- 48.Burk JA, Sarter M (2001): Dissociation between the attentional functions mediated via basal forebrain cholinergic and GABAergic neurons. Neuroscience. 105:899–909. [DOI] [PubMed] [Google Scholar]
- 49.Luchicchi A, Mnie-Filali O, Terra H, Bruinsma B, de Kloet SF, Obermayer J, et al. (2016): Sustained Attentional States Require Distinct Temporal Involvement of the Dorsal and Ventral Medial Prefrontal Cortex. Frontiers in neural circuits. 10:70–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGaughy J, Sarter M (1999): Effects of ovariectomy, 192 IgG-saporin-induced cortical cholinergic deafferentation, and administration of estradiol on sustained attention performance in rats. Behavioral neuroscience. 113:1216–1232. [DOI] [PubMed] [Google Scholar]
- 51.Witt ED, Mantione CR, Hanin I (1986): Sex differences in muscarinic receptor binding after chronic ethanol administration in the rat. Psychopharmacology (Berl). 90:537–542. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida T, Kuwabara Y, Sasaki M, Fukumura T, Ichimiya A, Takita M, et al. (2000): Sex-related differences in the muscarinic acetylcholinergic receptor in the healthy human brain--a positron emission tomography study. Ann Nucl Med. 14:97–101. [DOI] [PubMed] [Google Scholar]
- 53.Cosgrove KP, Esterlis I, McKee SA, et al. (2012): Sex differences in availability of β2*-nicotinic acetylcholine receptors in recently abstinent tobacco smokers. Archives of General Psychiatry. 69:418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jain A, Huang GZ, Woolley CS (2019): Latent Sex Differences in Molecular Signaling That Underlies Excitatory Synaptic Potentiation in the Hippocampus. The Journal of Neuroscience. 39:1552–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oberlander JG, Woolley CS (2016): 17β-Estradiol Acutely Potentiates Glutamatergic Synaptic Transmission in the Hippocampus through Distinct Mechanisms in Males and Females. The Journal of Neuroscience. 36:2677–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toulouse A, Nolan Y (2015): A role for mitogen-activated protein kinase phosphatase 1 (MKP1) in neural cell development and survival. Neural Regeneration Research. 10:1748–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pérez-Sen R, Queipo MJ, Gil-Redondo JC, Ortega F, Gómez-Villafuertes R, Miras-Portugal MT, et al. (2019): Dual-Specificity Phosphatase Regulation in Neurons and Glial Cells. International Journal of Molecular Sciences. 20:1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu JJ, Bennett AM (2005): Essential role for mitogen-activated protein (MAP) kinase phosphatase-1 in stress-responsive MAP kinase and cell survival signaling. Journal of Biological Chemistry. 280:16461–16466. [DOI] [PubMed] [Google Scholar]
- 59.Zhao Y, Wang S, Chu Z, Dang Y, Zhu J, Su X (2017): MicroRNA-101 in the ventrolateral orbital cortex (VLO) modulates depressive-like behaviors in rats and targets dual-specificity phosphatase 1 (DUSP1). Brain Research. 1669:55–62. [DOI] [PubMed] [Google Scholar]
- 60.Wang C-H, Zhang X-L, Li Y, Wang G-D, Wang X-K, Dong J, et al. (2015): Role of Hippocampus Mitogen-Activated Protein Kinase Phosphatase-1 mRNA Expression and DNA Methylation in the Depression of the Rats with Chronic Unpredicted Stress. Cellular and Molecular Neurobiology. 35:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


