Table 1: Unique domains and their associated pathological roles of (A) Transmembrane and (B) Secreted mucins.
A) Domains present in MUC1, MUC3A, MUC4, MUC12, MUC13, MUC15, MUC16, MUC17, and MUC21 mucins. While the interacting partners and downstream signaling of several mucin domains have been studied, many yet remain unknown (marked as *). Apart from the domains enumerated in the table, variable number tandem repeats (VNTRs) and transmembrane (TM) domains exist on each of these transmembrane mucins. VNTRs are the sites for glycosylation, and TM domains anchor these glycoproteins to the cell membrane. B) Several domains present in secreted mucins are characterized (107). However, the mechanistic contribution of these domains in cancer progression and metastasis is still elusive (marked as *) and may form the basis for future research.
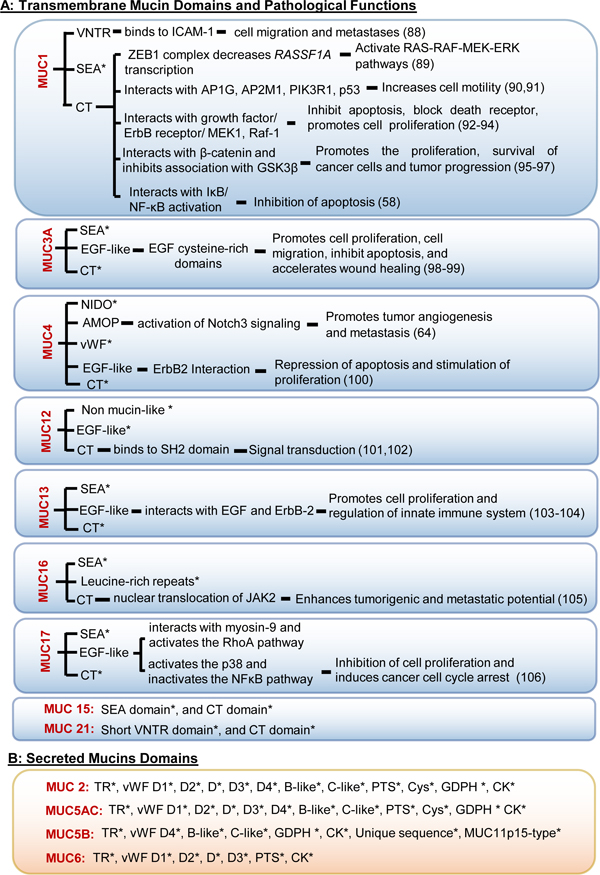 |
Abbreviations: AMOP: Adhesion-associated domain; CYS rich: Cysteine-rich domain; EGF repeats: EGF-like repeat region; NIDO: Nidogen-like domain; SEA: Sea urchin sperm receptor enterokinase agrin domain; vWF D1,2,3,4, B, C: Von Willebrand Factor type D1,2,3,4, B, C domains; PTS domain: Proline, threonine, serine-rich domains; CK domain: Cysteine Knot domain; GDPH: glycine-aspartic acid-proline-histidine.
