Abstract
Study objectives.
To determine whether insomnia patients with objective sleep disturbance are less responsive to cognitive and behavioral treatments than those without objective sleep disturbance, characterize effects of insomnia therapy on objective sleep, and determine whether reductions in nocturnal cognitive arousal correspond to changes in objective sleep.
Methods.
Secondary analysis of a single-site, randomized controlled trial. 113 postmenopausal women (56.40±5.34 years) with menopause-related insomnia disorder were randomized to 3 treatment conditions: cognitive-behavioral therapy for insomnia (CBTI), sleep restriction therapy (SRT), or sleep education control. Primary outcomes were the Insomnia Severity Index (ISI) and polysomnography (PSG) sleep parameters and were collected at pretreatment, posttreatment, and 6-month follow-up.
Results.
Patients with lower pretreatment PSG sleep efficiency had lower rates of insomnia remission after treatment relative to those with higher sleep efficiency (37.8% vs 61.8%). Neither CBTI and SRT produced clinically meaningful effects on PSG sleep. Exploratory analyses revealed that reductions in nocturnal cognitive arousal were associated with decreases in PSG sleep latency, but not wake after sleep onset.
Conclusions.
Our findings support an emerging literature suggesting that insomnia patients with objective sleep disturbance may have blunted response to insomnia therapy. Research is needed to enhance treatments to better improve insomnia in patients with objective sleep disturbance. A lack of observed CBTI and SRT effects on PSG sleep suggests that these therapies may be presently ill-designed to improve objective sleep. Nocturnal cognitive arousal may represent an entry point to improve objective sleep latency in insomnia.
Keywords: CBT-I, cognitive-behavioral therapy for insomnia, sleep restriction, cognitive arousal, rumination
Introduction
Polysomnography (PSG) is the gold standard tool for the objective quantification of sleep and assessing most sleep disorders.1 Yet, PSG has a complex history with insomnia in regard to its usefulness for evaluating, treating, and understanding the etiology of the disorder. This is reflected in diagnostic systems for sleep disorders (current and prior iterations of Diagnostic and Statistical Manual of Mental Disorders2 [DSM] and International Classification of Sleep Disorders3 [ICSD]) that define insomnia disorder as a symptom-based condition without PSG criteria. Weak empirical support for the diagnostic utility of PSG for insomnia led to recommendations against its use in routine evaluations, except to assess for other sleep disorders that can co-occur with or contribute to sleep complaints (e.g., sleep apnea).4–6 Evidence casting doubt on the utility of PSG in insomnia includes discrepancy between patient-reported sleep and objective sleep findings, little or no reliable difference in objective sleep between insomnia patients and healthy sleepers, and data suggesting that changes in objective sleep are unnecessary for successful alleviation of insomnia with therapy.7–9 Clinicians and researchers have thus considered patient-reported symptoms as a better indicator of the clinical experience of insomnia. Consequently, objective sleep assessment in insomnia has largely fallen by the wayside.
Although the insomnia patient population as a whole does not reliably exhibit objective sleep disturbances, a subset of the population does. Accumulating evidence suggests that insomnia disorder may be comprised of two broad phenotypes: insomnia with and without objective sleep disturbance.8,10,11 Even so, there is no universal consensus on how objective sleep disturbance should be operationalized in insomnia (e.g., total sleep time < 6 hrs, sleep latency and/or wake after sleep onset > 30 minutes, etc.). Nevertheless, the distinction is important as insomnia patients with objective sleep disturbances have poorer stress regulation, more comorbidities, and poorer long-term prognoses relative to those with normal objective sleep.10,11 As insomnia with objective sleep disturbance may have a different etiology and stronger biological basis than insomnia without objective sleep findings,10 researchers have taken interest in potential differences in treatment responsivity by phenotype.
These clinical trials focused on insomnia with vs without objective short sleep (<6 hrs total sleep time on PSG or actigraphy) and have produced mixed results. In two trials, insomnia patients with objective short sleep, relative to those with normal sleep duration, had blunted response to cognitive behavioral therapy for insomnia (CBTI),12,13 which is gold standard treatment for insomnia.14 To the contrary, three other trials showed no difference in treatment response between the normal sleep duration and short sleep insomnia phenotypes.15–17 As no clear patterns in methodology distinguish studies that support vs refute differential treatment response, it is unclear why such a degree of inconclusion with markedly opposing findings was produced. Although all studies used the total sleep time < 6 hrs operationalization for objective sleep disturbance, it is possible that a lack of established criteria for an objective sleep disturbance phenotype contributed in part to these mixed results; particularly as objective sleep disturbance may manifest in ways other than total sleep time < 6 hours.
Further complicating the matter of objective sleep disturbance and insomnia therapy, overwhelming evidence suggests that cognitive and behavioral interventions have minimal or no effect on objective sleep disturbance.9 As clinical trials of cognitive and behavioral treatments for insomnia have traditionally focused on patient-reported sleep symptoms, the lack of improvement in objective sleep and the potential importance of such effects has been downplayed. A recent review identified only five high quality CBTI trials that reported PSG outcomes, none of which required objective sleep criteria for trial entry.9 In-line with recent interest in objective sleep disturbance as an indicator of insomnia morbidity, mortality, and treatment-resistance,8,10–13 there has been renewed emphasis to better understand the effects of insomnia therapies on objective sleep and identifying how objective sleep outcomes can be improved.18
The present report was a secondary analysis of a single-site RCT comparing CBTI, sleep restriction therapy (SRT; a single component of CBTI and effective standalone treatment), and sleep hygiene education control (SHE) for the treatment of menopause-related DSM-52/ICSD-33 insomnia disorder in a sample of 150 postmenopausal women. See reports by Drake et al. and Kalmbach et al. for outcome data pertaining to insomnia, sleep diaries, depression, and daytime function.19–21 In this secondary analysis, we (1) tested pretreatment objective sleep disturbance as a potential moderator of treatment response, and (2) tested effects of CBTI and SRT on objective sleep disturbance relative to control. Based on proposals that insomnia with objective findings represents a more severe and biologically-based phenotype of the disorder, we hypothesized that patients with lower PSG sleep efficiency before treatment would report greater insomnia symptoms after CBTI and SRT relative to those with higher pretreatment sleep efficiency. We predicted no such pattern would be observed in the control condition. Importantly, we chose to test sleep efficiency as a moderator as it captures all manifestations of objective nocturnal wakefulness (sleep latency, wake after sleep onset, short sleep duration) and because no universally accepted cutoff defining objective sleep disturbance in insomnia exists.
Second, a lack of objective sleep criteria for clinical trial entry has been identified as a limitation of prior RCTs evaluating CBTI effects on objective sleep due to restricted range and potential insufficient statistical power to detect effects. The present RCT required objective sleep disturbance for entry. When this study was initially proposed, our team hypothesized that CBTI and SRT would improve objective sleep. However, meta-analytic data published in the interim9 changed our expectations, thus we predicted that neither CBTI nor SRT would improve objective sleep relative to control, despite our PSG inclusion criteria.
In an exploratory analysis based on data from our lab and others showing that cognitive arousal and rumination is associated with objective sleep disturbance,22–27 we conducted posthoc analyses to investigate whether changes in nocturnal cognitive arousal were associated with changes in PSG sleep latency and wake after sleep onset, irrespective of treatment condition (as we hypothesized no treatment effects). This exploratory analysis was conducted in attempt to identify therapeutic targets associated with changes in objective sleep.
Methods
Subjects and setting
This trial was conducted in a 6-hospital health system in metro Detroit, Michigan, USA. Patients were recruited from the health system in primary care and sleep medicine clinics, from the community via newspaper advertisements, and from a database of prior sleep center studies. Inclusionary criteria included having 12 consecutive months without menses and self-reported wake after sleep onset ≥ 1 hour per night at least 3 nights per week, as well as meeting criteria for DSM-5 insomnia disorder that onset or exacerbated during the peri- or postmenopausal period per clinical interview with a registered nurse with specialty training in behavioral sleep medicine. Inclusionary criteria regarding objective sleep disturbance required women exhibit mean wake after sleep onset of 45 minutes or more on 2 overnight polysomnography (PSG) studies (adaptation night + baseline night, neither of which could have wake after sleep onset < 30 minutes). Exclusionary criteria included prior or current DSM-5 major depression per diagnostic interview, sleep-wake disorders other than insomnia (examined on PSG adaptation night and per patient report), and medications influencing sleep. Women receiving hormone replacement therapy were eligible to participate.
Participants were randomized to 1 of 3 conditions: sleep hygiene education control (SHE), sleep restriction therapy (SRT), or cognitive-behavioral therapy for insomnia (CBTI). Randomization was conducted using 150 allocations (50 per group) that were ordered randomly and concealed in envelopes. Group allocation for each participant was then assigned using the order of concealed envelopes. While double-blind could not be achieved given the nature of the behavioral interventions, subjects were not informed which treatments were considered control versus active, or of the specific hypotheses. Assessments of self-reported insomnia symptoms (via online surveys) and objective sleep parameters (via in-lab overnight polysomnography) were collected prior to treatment, at posttreatment (within 2 weeks of completing treatment), and 6 months after treatment completion.
Polysomnography assessments and schedule
The study protocol included 4 overnight laboratory PSG studies. Night 1: Subjects who passed the in-person screening interview then underwent a PSG adaptation/screening night with a montage routinely used to rule out sleep disorders other than insomnia disorder such as obstructive sleep apnea and periodic limb movement disorder (apnea-hypopnea index > 10, periodic limb movement index with arousal > 10). Night 2: A week later, patients underwent a second PSG night, which was used to help determine eligibility (see Subjects and setting section above) and served as the pretreatment baseline night to which posttreatment and follow-up data would be compared. Night 3: Within 2 weeks of completing treatment, subjects underwent a third PSG study to record posttreatment objective sleep disturbance. Night 4: Six months later, subjects underwent the final PSG study to record longer-term objective sleep. For all subjects, overnight PSG study bedtimes were scheduled in accordance with self-reported habitual bedtimes and ended 8 hours after lights out.
Treatment Conditions
Sleep hygiene education (SHE), i.e., minimal intervention control condition.
Women randomized to the online sleep hygiene education condition received 6 weekly emails including general, non-personalized information on the following topics: the basics of endogenous sleep regulation; the impact of sleep on health problems such as obesity, diabetes, and hypertension; the effects of stimulants and other sleep-disruptive substances; the relationship between sleep, diet, and exercise; and tips on creating a sleep-conducive bedroom environment. Sleep hygiene is neither the primary cause nor a sufficient therapeutic target in insomnia disorder and therefore served as an ideal minimal intervention control condition and real-world comparator.28
Cognitive-behavioral therapy for insomnia (CBTI) was 1 of 2 active treatments in this RCT. Women randomized to CBTI completed 6 face-to-face sleep therapy sessions with a registered nurse who specializes in behavioral sleep medicine. CBTI is a structured, multi-modal treatment that targets sleep-disruptive behaviors and beliefs (see Perlis et al. 200629). Data from clinical trials consistently show that CBTI is as efficacious as pharmacological treatment in the short-term, but produces superior treatment response in the long-term.14,30 CBTI patients received 6 weekly sessions, which covered behavioral (sleep restriction and stimulus control) and cognitive (e.g., cognitive restructuring) components, as well as relaxation strategies (e.g., progressive muscle relaxation and autogenic training) and sleep hygiene education.
Sleep restriction therapy (SRT) was the 2nd active treatment in this RCT and is considered an effective standalone behavioral treatment for insomnia.31 Although SRT actually predates CBTI, SRT is now commonly packaged as part of CBTI and is considered a critical element. As CBTI consists of SRT plus multiple other components, SRT is the briefer of the two interventions. Here, SRT was delivered as a 2-week intervention. Specifically, the initial face-to-face session consisted of reviewing patient sleep history, education and rationale for sleep restriction practices, and behavioral homework. Then 4 follow-up sessions (3 phone contacts, each 3–4 days apart, followed by a 2nd face-to-face session) were delivered across the following 2 weeks and were used to titrate sleep schedules based on sleep diary data. SRT as delivered in this RCT was the same as the sleep restriction component in CBTI, except that sessions were spaced more closely together (every 3–4 days rather than approximately weekly), and thus prescribed sleep schedules were based on fewer days of sleep diary data.
Measures
Polysomnography (PSG) sleep parameters/nocturnal wakefulness.
PSG data were scored by a certified sleep technician according to standard procedures by the American Academy of Sleep Medicine’s 2012 guidelines.32 PSG scorers were blind to group. Sleep measures included sleep latency (minutes from lights out to first epoch of sleep), wake after sleep onset (minutes awake post onset), sleep efficiency (proportion of time in bed asleep), and total sleep time (sleep duration).
All self-report measures were collected via online surveys hosted by Qualtrics, LLC. Prior to treatment, patients reported sociodemographic and health history information.
The Insomnia severity index (ISI) is a 7-item self-report measure of insomnia symptom severity.33 Scores range from 0 to 28 with higher scores indicating greater symptom severity. The ISI was administered at pretreatment, posttreatment, and 6-month follow-up. Recommendations for clinical trials research include detecting positive insomnia cases based on ISI scores ≥ 11, whereas remission is indicated by ISI scores ≤ 7.34
The Presleep Arousal Scale’s Cognitive factor (PSAS-C)35 was used to measure cognitive arousal during the presleep period, i.e., when individuals are in bed attempting to fall asleep. The PSAS Cognitive scale consists of 8 items (e.g., ‘review or ponder events of the day’ and ‘can’t shut off your thoughts’) and possible scores range from 8 to 40 with higher scores indicating greater presleep cognitive arousal.
Presleep somatic arousal was measured using the Presleep Arousal Scale’s Somatic factor (PSAS-S).35 The PSAS Somatic scale consists of 8 items (e.g., ‘heart racing, pounding, or beating irregularly’ and ‘a tight, tense feeling in your muscles’) and possible scores range from 8 to 40 with higher scores indicating greater presleep cognitive arousal.
Analysis Plan
Analyses were conducted using SPSS version 25. Age, menopause-related characteristics, and pretreatment PSG sleep parameters were first presented and cross-sectionally compared across the 3 treatment conditions to identify group differences before treatment. Next, we examined whether pretreatment objective sleep disturbance as measured by PSG sleep efficiency moderated treatment response. Specifically, we ran repeated measures analysis of covariance (ANCOVA) models to test changes in ISI from pre to posttreatment/follow-up with Treatment (active treatment [CBTI and SRT] vs control) as a between group factor and pretreatment sleep efficiency as a covariate. Notably, CBTI and SRT were combined into a single group, because our previously published findings show that they are both efficacious treatments36 and our primary focus here is on PSG Sleep Efficiency as a moderator of treatment response, irrespective of modality. Thus, results from this model showed interactions for Treatment X Time (to demonstrate that active treatment reduced ISI greater than control, which we have reported in greater detail elsewhere36) and for PSG sleep efficiency X Time to evaluate whether pretreatment objective sleep disturbance altered trajectory of symptoms over time, irrespective of condition. Significant interactions were then deconstructed with posthoc independent samples and paired samples t-tests to describe the observed effects in relation to active treatment vs control.
We then tested treatment effects on objective sleep disturbance (i.e., PSG-determined sleep latency, wake after sleep onset, sleep efficiency, and total sleep time). We first ran 3×2 repeated measures ANOVA models to examine Treatment x Time interactions for changes in PSG sleep parameters from pretreatment to posttreatment/follow-up. Any significant Treatment X Time interactions were followed by paired samples t-tests within each condition to test for potential simple effects. In addition, a cross-sectional one-way ANOVA was used to compare mean levels for each treatment outcome to determine differences in symptom levels across groups at posttreatment and 6-month follow-up. Lastly, we used multivariate linear regression models to explore whether changes in presleep cognitive and somatic arousal were associated with acute changes in objective sleep. Specifically, we regressed changes in PSG sleep parameters between pre and posttreatment on changes in presleep cognitive and somatic arousal.
Results
Participant enrollment and attrition.
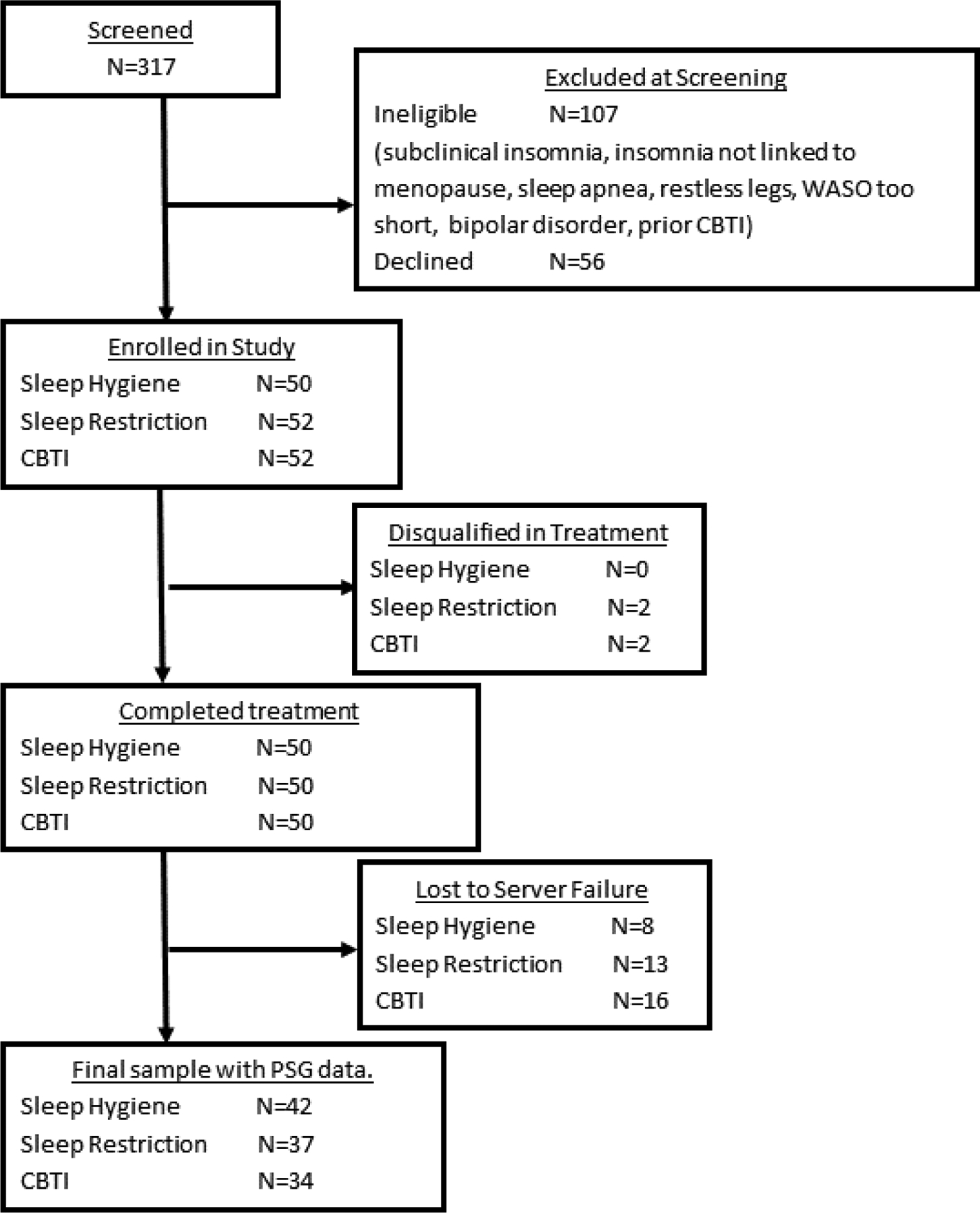
Refer to Figure 1 flow chart of study enrollment and participation. A total of 317 postmenopausal women were screened for eligibility. Of these individuals, 107 women were ineligible and another 56 declined to participate or had scheduling conflicts. Thus, 154 postmenopausal women were randomized to treatment: SHE: N=50, SRT: N=52, and CBTI: N=52. Two subjects in both the SRT and CBTI conditions were disqualified during treatment for changes in medication or new onset comorbid sleep disorder. Thus, recruitment included two more individuals to replace those who were disqualified. This resulted in 50 subjects completing treatment in each of the 3 conditions. All 150 subjects provided self-reported posttreatment outcome data, whereas 16% of treatment completers did not provide follow-up data 6 months later (Figure 1). Regarding PSG data, 147 women completed all 3 overnight PSGs (pretreatment, posttreatment, follow-up). However, data from several subjects were lost due to a shared network server failure, thus PSG data from 113 subjects were available for analysis. Because the present study focused on objective sleep disturbance, all data reported herein are from the 113 patients with partial or complete PSG data.
Figure 1.
Patient flowchart for recruitment and study protocol.
Screening and sample characteristics
See Table 1 for sample demographics for the full sample and by treatment condition. Our sample was largely comprised of non-Hispanic White and non-Hispanic Black women. Regarding objectively estimated sleep, mean PSG sleep latency was within normal limits (11.95±13.48 m), whereas mean PSG wake after sleep onset was high (78.97±44.29 m), sleep efficiency was low (81.19±9.98), and total sleep time was relatively short (390.59±46.78 minutes). One-way ANOVA models revealed patient did not differ on age or pretreatment ISI (Tables 1). To determine whether pretreatment PSG sleep efficiency was associated with patient-reported insomnia severity, we compared patients with pretreatment PSG sleep efficiency < 85% vs ≥ 85%, which did not differ in pretreatment ISI scores (14.60±3.58 vs 15.51±4.78, t[110]=1.15, p=.25).
Table 1.
Sample characteristics prior to treatment (n=113).
| All subjects | SHE | SRT | CBTI | ||
|---|---|---|---|---|---|
| Sample size | 113 | 42 | 37 | 34 | |
| Age (M±SD) | 56.40±5.34y | 57.21±5.30 | 56.68±5.18 | 55.09±5.46 | F(2,110)=1.58, p=.21 |
| Race (n;%) | |||||
| White | 56; 49.6% | 20; 47.6% | 21; 56.8% | 15; 44.1% | |
| Black | 51; 45.1% | 20; 47.6% | 13; 35.1% | 18; 54.5% | |
| Hispanic or Latinx | 1; 0.9% | -- | 1; 2.7% | -- | |
| Not reported | 5; 4.4% | 2; 4.8% | 2; 5.4% | 1; 2.9% | |
| Years since last menses (M±SD) | 7.19±7.24 | 7.62±8.01 | 6.42±6.62 | 7.51±7.06 | F(2,110)=.29, p=.75 |
| Hormone Replacement Therapy (n;%) | 3; 2.7% | 2; 4.8% | 1; 2.7% | 0; 0.0% | χ2=1.65, p=.44 |
| Medical/Surgical Menopause (n;%) | 27; 23.9% | 7; 16.7% | 9; 24.3% | 11; 32.4% | χ2=2.55, p=.28 |
| ISI, prior to treatment (M±SD) | 15.04±4.15 | 14.86±4.45 | 15.16±3.80 | 15.12±4.23 | F(2,110)=.06, p=.94 |
Note: SHE = sleep hygiene education control. SRT = sleep restriction therapy. CBTI = cognitive behavioral therapy for insomnia. PSG = polysomnography. m = minutes. Sleep latency = minutes from lights out to first epoch of sleep. Wake after sleep onset = minutes awake post sleep onset. Sleep efficiency = proportion of time in bed spent asleep X 100. Total sleep time = minutes spent asleep during total PSG recording. F = F-statistic for one-way analysis of variance to compare means across SHE, SRT, and CBTI groups. χ2 = chi-square statistic to compare proportions across SHE, SRT, and CBTI groups. p = significance vale.
Pretreatment objective sleep disturbance buffers treatment response to insomnia therapy
Acute treatment response.
We first ran a repeated measures ANCOVA model examining changes in ISI from pre to posttreatment with Treatment (active treatments, i.e., CBTI and SRT, vs control) as a between group factor and baseline PSG sleep efficiency as a covariate. The Treatment X Time interaction was significant (F[1,108)=51.43, p<.001) and a posthoc paired-samples t-test showed that patients who received active treatment reported a mean decrease of 7.5 points on the ISI (t[70]=−12.24, p<.001), whereas mean ISI decreased by just 1.2 points for controls.
The Treatment X PSG Sleep Efficiency interaction was also significant (F[1,108]=10.64, p=.001) such that patients with lower pretreatment sleep efficiency exhibited smaller improvements in ISI by the end of treatment. An independent samples t-test showed that posttreatment ISI scores were higher for patients with high pretreatment sleep efficiency compared to those with low sleep efficiency (8.92±4.13 vs 6.29±4.24, t[70]=2.64, p=.01, Cohen’s d=.66).
We then evaluated treatment outcomes related to objective sleep disturbance within active treatment and then within the control condition. Among patients receiving active treatment, pretreatment sleep efficiency was negatively correlated with change scores in ISI such that lower sleep efficiency was associated with smaller decreases in symptoms (Table 2). Notably, mean ISI scores did not differ before treatment between patients with high vs low sleep efficiency (Table 3). However, after active treatment, posthoc descriptive comparisons showed that patients with high pretreatment sleep efficiency, relative to those with low sleep efficiency, reported lower insomnia symptom severity and higher rates of remission (Table 3). Specifically, patients in active treatment with high pretreatment sleep efficiency had a mean reduction of 9.2 points on the ISI, whereas patients with low efficiency only reported an ISI reduction of 5.9 points, (t[70]=2.79, p=.007, Cohen’s d=.68). This suggests that response to insomnia therapies for patients with objective sleep disturbance is ~33% lower than response for patients without objective sleep findings. This pattern was not replicated in the control condition (Tables 2 and 3). See Supplementary Table 1 for pre and posttreatment mean ISI scores for patients with high and low sleep efficiency across all 3 conditions.
Table 2.
Correlations between pretreatment sleep efficiency and changes in insomnia symptom severity among patients in active treatment and in the control condition.
| ISIT2-T1 | ISIT3-T1 | |
|---|---|---|
| Active Treatment | ||
| Pretreatment SE | −.37** | −.29* |
| Control Condition | ||
| Pretreatment SE | −.10 | −.07 |
Note: Active treatment includes CBTI and SRT participants. Control condition includes sleep hygiene education control participants. ISIT2-T1 = change in insomnia severity index score from pretreatment to posttreatment. ISIT3-T1 = change in insomnia severity index score from pretreatment to 6-month follow-up. SE = PSG-defined sleep efficiency.
Table 3.
Descriptive comparisons of insomnia symptom severity and remission rates across treatment between patients with and without objective sleep disturbance (Mean ± Standard Deviations presented).
| Pretreatment | Posttreatment | 6-Month Follow-up | |||
|---|---|---|---|---|---|
| Patients in active treatment. | |||||
| ISI | Symptom Severity | Symptom Severity | Remission Rates | Symptom Severity | Remission Rates |
| t(69)=−.67, p=.51 | t(69)=2.64, p.01, d=.63 | χ2=4.06, p=.04, RR=1.63 | t(63)=1.55, p=.13 | χ2=3.28, p=.07 | |
| SE≥85% | 15.47±4.44 | 6.29±4.24 | 21/34; 61.8% | 6.63±4.99 | 22/30; 73.3% |
| SE<85% | 14.84±3.56 | 8.92±4.13 | 14/37; 37.8% | 8.54±4.94 | 18/35; 51.4% |
| Patients in control condition. | |||||
| ISI | Symptom Severity | Symptom Severity | Remission Rates | Symptom Severity | Remission Rates |
| t(39)=−.92, p.36 | t(39)=.20, p.84 | χ2=−16, p=.69 | t(35)=−.34, p.74 | χ2=69, p=.41 | |
| SE≥85% | 15.60±5.63 | 13.40±5.01 | 1/26; 3.8% | 13.54±6.17 | 3/24; 12.5% |
| SE<85% | 14.27±3.66 | 13.69±4.07 | 1/15; 6.7% | 12.96±4.19 | 3/13; 23.1% |
Note: Active treatment includes CBTI and SRT participants. Control condition includes sleep hygiene education control participants. ISI = insomnia severity index. t = t-statistic for independent samples t-test. p = significance value. χ2 = chi-square. SE = sleep efficiency. SE≥85% = high sleep efficiency before treatment reflecting no objective sleep disturbance. SE<85% = low sleep efficiency before treatment reflecting objective sleep disturbance.
Long-term treatment response.
A repeated measures ANCOVA examining changes in ISI from pretreatment to 6-month follow-up again showed that the Treatment X Time interaction was significant (F[1,109)=54.25, p<.001). In addition, the Treatment X Sleep Efficiency interaction was also significant (F[1,99]=5.14, p=.02) such that lower pretreatment sleep efficiency predicted smaller gains in insomnia improvement through 6-month follow-up.
Among those in active treatment, pretreatment sleep efficiency was associated with longer-term reductions in ISI such that lower objective sleep disturbance before treatment was associated with greater reductions in ISI over the long-term (Table 2). Even so, posthoc descriptive comparisons showed that ISI scores did not differ between patients with high or low sleep efficiency at 6-month follow-up, although remission rates approached significance (Table 3). In the control condition, pretreatment sleep efficiency was once again not associated with changes in ISI scores nor clinical outcomes at 6-month follow-up (Tables 2 and 3). See Supplementary Table 1 for follow-up mean ISI scores for patients with high and low sleep efficiency across all 3 conditions.
CBTI vs SRT vs SHE control treatment effects on objective sleep disturbance
Sleep latency.
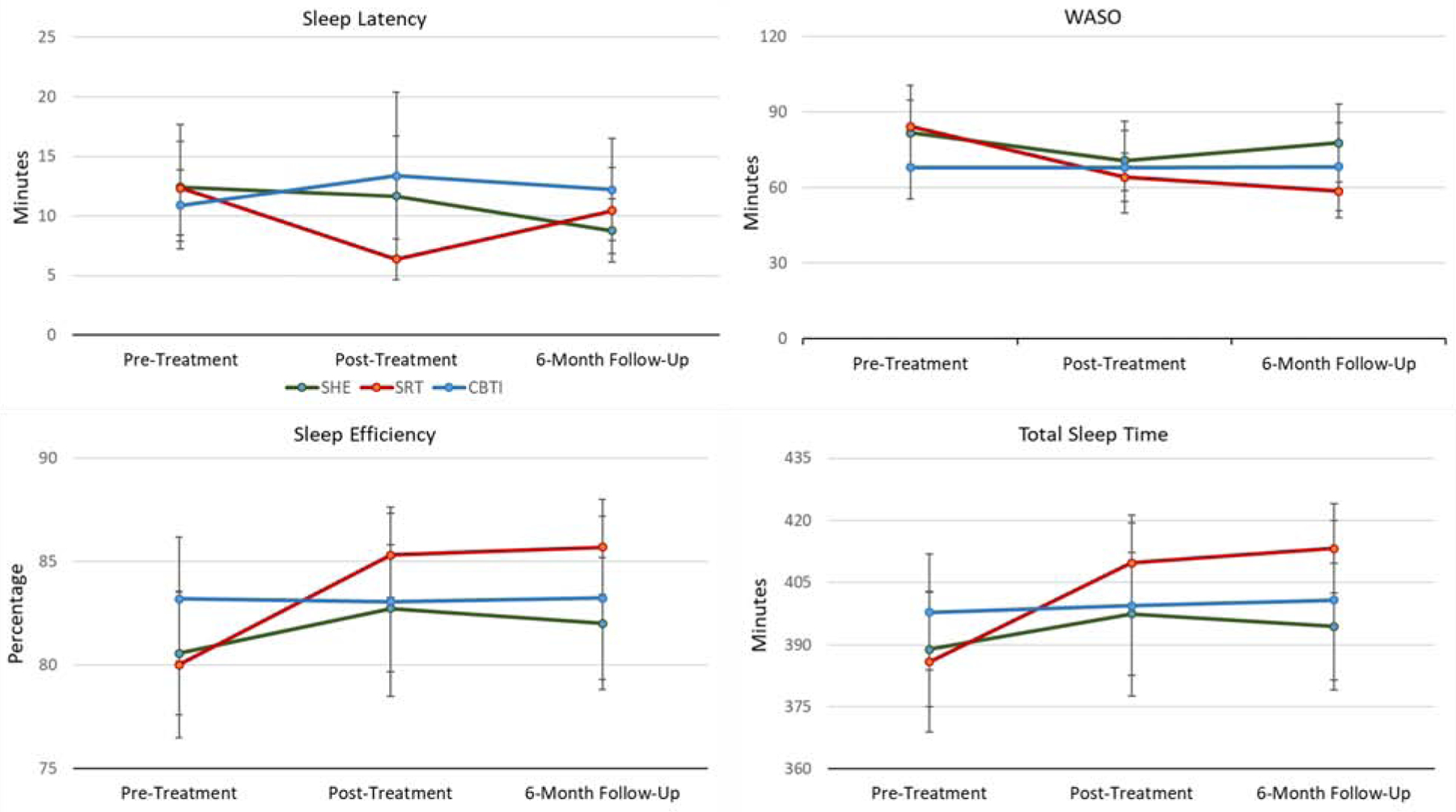
See Table 4 for sleep latency means and standard deviations for all groups at posttreatment and 6-month follow-up. Also see Figure 2 for visual representation of data. When testing acute treatment effects, a repeated measures ANOVA showed that sleep latency did not significantly change for the full sample between pre and posttreatment (F[1,106]=.82, p=.37). However, a significant Treatment X Time interaction was observed (F[2,106]=3.23, p=.04). We then ran series of posthoc paired samples t-tests to identify group(s) wherein sleep latency significantly changed during treatment. In the SRT group, sleep latency significantly decreased by 5 minutes (t[33]=3.24, p=.003). To the contrary, no significant within-group changes in sleep latency were observed for CBTI (p=.27) or SHE (p=.74). Despite the significant reduction in sleep latency in the SRT group, a one-way ANOVA revealed no posttreatment group differences in sleep latency (Table 4).
Table 4.
Comparing CBTI vs SRT vs sleep hygiene education on objective sleep parameters.
| Pretreatment Group Comparisons | Posttreatment Group Comparisons | 6-month Follow-up Group Comparisons | |
|---|---|---|---|
| Sleep latency | F(2,110)=.15, p=.86 | F(2,108)=1.82, p=.17 | F(2, 108)=1.02, p=.36 |
| SHE | 12.45±17.33 m | 11.66±16.78 m | 8.77±6.83 m |
| SRT | 12.35±12.21 m | 6.38±5.30 m | 10.44±11.23 m |
| CBTI | 10.88±8.92 m | 13.37±20.91 m | 12.22±12.75 m |
| Wake after sleep onset | F(2,110)=1.09, p=.34 | F(2,108)=.24, p=.79 | F(2, 108)=1.58, p=.21 |
| SHE | 81.82±42.95 m | 70.80±39.54 m | 77.77±51.04 m |
| SRT | 84.25±.51.15m | 64.17±29.83 m | 58.54±32.94 m |
| CBTI | 67.97±37.15 m | 68.06±54.26 m | 68.34±51.95 m |
| Sleep efficiency | F(2,11)=1.02, p=.36 | F(2,108)=.64, p=.56 | F(2, 108)=1.28, p=.28 |
| SHE | 80.58±9.88 | 82.74±10.16 | 82.01±10.58 |
| SRT | 80.01±10.94 | 85.31±6.34 | 85.71±7.15 |
| CBTI | 83.20±8.94 | 83.08±13.61 | 83.25±11.78 |
| Total sleep time | F(2,110)=.62, p=.54 | F(2,108)=.62, p=.54 | F(2, 108)=1.43, p=.24 |
| SHE | 388.92±45.98 m | 397.46±48.97 m | 394.38±50.65 m |
| SRT | 385.84±52.23 m | 409.68±30.42 m | 413.25±33.62 m |
| CBTI | 397.82±41.72 m | 399.43±65.19 m | 400.74±57.20 m |
Note: SHE = sleep hygiene education control. SRT = sleep restriction therapy. CBTI = cognitive behavioral therapy for insomnia. PSG = polysomnography. m = minutes. Sleep latency = minutes from lights out to first epoch of sleep. Wake after sleep onset = minutes awake post sleep onset. Sleep efficiency = proportion of time in bed spent asleep X 100. Total sleep time = minutes spent asleep during total PSG recording. F = F-statistic for one-way analysis of variance to compare means across SHE, SRT, and CBTI groups. p = significance vale.
Figure 2.
Pretreatment, posttreatment, and follow-up PSG sleep latency, wake after sleep onset, sleep efficiency, and total sleep time data for CBTI, SRT, and SHE (means and standard errors presented).
A repeated measures ANOVA also showed that PSG sleep latency did not significantly change from pretreatment to 6-month follow-up (F[1,104]=2.26, p=.14) nor was a Treatment X Time interaction observed (F[2,104]=.85, p=.43). Consistent with these null findings, a one-way ANOVA revealed no group differences in sleep latency 6 months after treatment.
Wake after sleep onset.
See Table 4 for wake after sleep onset means and standard deviations for all groups at posttreatment and 6-month follow-up. Also see Figure 2 for visual representation of data. When exploring acute changes in sleep maintenance, a repeated measures ANOVA revealed that PSG wake after sleep onset decreased by 11 minutes for the full sample, which was a significant reduction (F[1,106]=5.86, p=.02). Despite this acute reduction in wake after sleep onset, no Treatment X Time interaction was observed (F[2,106]=1.08, p=.34). Consistent with this null interaction finding, a one-way ANOVA revealed no posttreatment group differences in PSG wake after sleep onset (p=.79; Table 4).
Findings from the 6-month follow-up data were consistent with acute models. A repeated measures ANOVA again showed that wake after sleep onset decreased by 11 minutes from pretreatment to 6-month follow-up (F[1,104]=4.19, p=.04). Yet, once again, the Treatment X Time interaction was non-significant (F[2,104]=2.49, p=.09) and no group differences in wake after sleep onset were observed (p=.21; Table 4).
Sleep efficiency.
See Table 4 and Figure 2 for PSG sleep efficiency results. Repeated measures ANOVA models revealed no acute changes in sleep efficiency (F[1,106]=2.25, p=.11), whereas PSG sleep efficiency increased by ~2% for the full sample by 6-month follow-up (F[1,104]=5.63, p=.02). Even so, no Treatment X Time interaction was observed for acute (p=.11) or long-term (p=.09) effects. No treatment condition differences in sleep efficiency were observed at posttreatment (p=.54) or 6 months later (p=.24; Table 4).
Total sleep time.
As time in bed was fixed, results for total sleep time therefore mirror sleep efficiency data (Table 4, Figure 2). Thus, we will simply acknowledge that no Treatment X Time interactions were observed and no group differences at posttreatment or 6-month follow-up were significant.
Exploring associations between presleep arousal and objective sleep disturbance.
Prior to treatment, multivariate linear regression showed that PSG sleep latency was significantly associated with presleep cognitive arousal, but not with presleep somatic arousal (Table 5). Neither cognitive nor somatic arousal was associated with PSG wake after sleep onset. Posttreatment data replicated pretreatment data such that PSG sleep latency was significantly associated with presleep cognitive arousal, whereas somatic arousal was nonsignificant (Table 5). Posttreatment PSG wake after sleep onset was not associated with cognitive nor somatic arousal.
Table 5.
Associations of presleep cognitive and somatic arousal with objective sleep disturbance.
| b | β | p | b | β | p | ||
|---|---|---|---|---|---|---|---|
| Pretreatment Sleep latency | Pretreatment Wake after sleep onset | ||||||
| Pretreatment PSAS-C | .90 | .26 | .01 | Pretreatment PSAS-C | −.27 | −.02 | .83 |
| Pretreatment PSAS-S | .06 | .03 | .74 | Pretreatment PSAS-S | −.83 | −.14 | .19 |
| Posttreatment Sleep latency | Posttreatment Wake after sleep onset | ||||||
| Pretreatment PSAS-C | 1.89 | .50 | .001 | Pretreatment PSAS-C | 1.37 | .12 | .42 |
| Pretreatment PSAS-S | −.44 | −.18 | .19 | Pretreatment PSAS-S | −.23 | −.03 | .83 |
| Δ Sleep latency | Δ Wake after sleep onset | ||||||
| Δ PSAS-C | 1.34 | .28 | .04 | Δ PSAS-C | .26 | .02 | .89 |
| Δ PSAS-S | −.17 | −.06 | .64 | Δ PSAS-S | −.19 | −.02 | .86 |
Note: b = unstandardized regression coefficient. β = standardized regression coefficient. p = statistical significance. PSAS-C = presleep arousal scale, cognitive factor. PSAS-S = presleep arousal scale, somatic factor. Sleep latency = minutes from lights out to first epoch of sleep. Wake after sleep onset = minutes awake post sleep onset. Δ Sleep latency = Change in PSG-based sleep latency from pre to posttreatment. Δ Wake after sleep onset = Change in PSG-based wake after sleep onset from pre to posttreatment. Δ PSAS-C = Change in presleep arousal scale cognitive factor scores from pre to posttreatment. Δ PSAS-S = Change in presleep arousal scale somatic Factor scores from pre to posttreatment.
Lastly, we conducted two multivariate linear regression models to test whether acute improvements in presleep arousal were associated with improvements in objective sleep (Table 5). Consistent with pre and posttreatment models, reductions in PSG sleep latency were significantly associated with reductions in presleep cognitive arousal, but not with changes in somatic arousal. Changes in wake after sleep onset were not associated with changes in cognitive nor somatic arousal.
Discussion
In postmenopausal women with chronic insomnia and PSG-defined objective sleep maintenance difficulties, patients with greater objective sleep disturbance prior to treatment reported smaller gains on self-report measures of insomnia symptoms after therapy, relative to patients with less objective sleep disturbance. These data suggest that insomnia patients with objective sleep disturbance may respond more poorly to cognitive and behavioral insomnia treatments than patients without objective sleep findings. Not only did objective sleep disturbance appear to blunt treatment response, but CBTI and SRT exerted no therapeutically beneficial effects on objective sleep disturbance relative to control. Some evidence suggested that sleep latency could potentially be reduced with sleep restriction, but it is unclear whether this effect would be durable or clinically meaningful. Even so, patients who reported decreases in nocturnal cognitive arousal also exhibited reductions in sleep latency on PSG, which suggests that nocturnal cognitive arousal might represent an entry point for cognitive-behavioral interventions to reduce objective sleep latency in patients presenting with trouble falling asleep.
Objective sleep disturbance as a moderator of treatment response.
Patients with lower PSG sleep efficiency before treatment had poorer acute and long-term self-reported insomnia outcomes relative to insomnia patients without objective findings. Important to emphasize here is that low PSG sleep efficiency before treatment was not associated with pretreatment patient-reported insomnia severity, thus pretreatment objective sleep disturbance is not merely a proxy for patient-reported illness severity. Among patients receiving CBTI or SRT, those with greater objective sleep disturbance had smaller acute and long-term insomnia improvements and lower rates of remission after therapy relative to those without objective sleep difficulties. In the control group, treatment outcomes did not differ as a function of pretreatment objective sleep disturbance. These results are consistent with prior reports suggesting that objective short sleep blunts response to CBTI,12,13 but contrary to reports that show no difference in insomnia therapy outcomes between insomnia patients with normal vs short objective sleep duration.15–17
Reasons why our study supported objective sleep disturbance as a moderator of treatment outcomes in insomnia—as findings in the field are highly mixed—are not readily clear, but we can speculate based on notable differences in our methodology. One potential explanation is that our entry criteria included minimal requirements for objective wake after sleep onset. Prior CBTI trials with PSG outcomes have not required objective nocturnal wakefulness and may have thus suffered from restriction of range.9 In addition, our primary analyses for examining objective sleep disturbance as a moderator centered on sleep efficiency as a continuous variable, rather than dichotomizing the sample into two subgroups. Preserving the dimensional aspect of objective sleep disturbance may have retained sufficient statistical power to detect moderating effects of objective sleep disturbance on treatment outcomes, which may have been lost if we tested our hypotheses by dichotomizing the sample into two subgroups (as observed in our posthoc descriptive comparisons between patients with high vs low sleep efficiency based on the <85% cutoff). This is especially critical in the absence of empirically supported PSG sleep efficiency-based clinical cutoff criteria for phenotypes.
Operationalizing objective sleep disturbance in insomnia: The need to identify a clinical indicator.
Our reasoning behind operationalizing objective sleep disturbance as pretreatment sleep efficiency on PSG was twofold: (1) sleep efficiency is encompassing in that it captures sleep latency, wake time during sleep, and early morning awakenings, and (2) no clear cutoff defining objective sleep disturbance exists in the literature, thus examining sleep efficiency as a continuous variable at present may be preferable. Important to emphasize here is that this study’s posthoc group comparisons based on < 85% sleep efficiency were for descriptive purposes and in alignment with clinical thresholds in behavioral sleep medicine used to guide decisions in therapy protocol, but are not intended to elucidate phenotypes.
Indeed, no consensus PSG criteria have been firmly established to classify the objective sleep disturbance phenotype. A lack of standardization is reflected by myriad operationalizations of objective sleep disturbance in insomnia research (e.g., < 85% sleep efficiency, < 6.5 hrs total sleep time, > 30 mins sleep latency, etc.).8 Recently, empirical support for short objective sleep duration (< 6 hrs total sleep time, ideally assessed via PSG) as a robust indicator of objective sleep disturbance in insomnia has flourished,10,11,37–39 yet it is unclear whether other PSG criteria can also identify insomnia patients with other distinct and clinically meaningful patterns of objective sleep disturbance.
Although insomnia with short sleep duration has garnered much deserved attention, it is possible that short sleep is just one clinically meaningful indicator of objective sleep disturbance. If other indicators of significant objective sleep disturbance exist, then it is possible that a lack of consideration for these other potential manifestations of objective sleep disturbance in insomnia have contributed to mixed findings in the field. Along these lines, objective short sleep reflects physiologic hyperarousal in the context of insomnia. It is thus critical to determine whether physiologic hyperarousal underlies other forms of objective sleep disturbance (prolonged latency, extended wake after sleep onset, etc.). Future large-scale research is needed to identify valid and reliable objective criteria to differentiate between insomnia patients with and without objective findings, potentially in addition to the widely supported short sleep phenotype, and to characterize the psychological and neurobiological underpinnings, as well as characterize morbidity, mortality, and treatment-responsivity related to any indicators of objective sleep disturbance in insomnia. If objective findings are clearly established and refined, then clinical trials research will be better positioned to determine whether triage based on objective criteria can improve treatment outcomes.
Objective sleep disturbance as a treatment outcome in insomnia therapy.
A notable strength of our RCT over prior trials is that we required objective sleep disturbance per PSG for study entry. Despite oversampling insomnia patients with objective findings, we did not observe any meaningful effects of CBTI or SRT on objective sleep at posttreatment or 6-month follow-up, relative to control. Our findings, in combination with results from a recent meta-analysis of five high quality clinical trials,9 suggest that insomnia therapies may not exert changes to objective sleep disturbance in a clinically meaningful way. Yet, there is ample opportunity in future research to augment CBTI and SRT to enhance treatment effects of psychological intervention on objective nocturnal wakefulness.
Indeed, although evidence suggests that insomnia and objective sleep disturbance are most toxic when co-occurring,10,11 objective sleep disturbances have nevertheless been linked to negative health consequences including depression and increased perceived stress, obesity and obesogenic behaviors, and cardiometabolic dysregulation.40–43 Thus, enhancing insomnia therapies to better improve sleep in insomnia patients with these objective findings is an important endeavor for clinical sleep research, especially given growing evidence that patients with objective sleep may not experience adequate alleviation of self-reported symptoms with insomnia therapy. Research is needed to identify therapeutic targets that may serve as entry points to therapeutically and durably improve objective sleep through psychological or pharmacological interventions.
Can reducing nocturnal cognitive arousal improve objective sleep disturbance?
Prior and after treatment, nocturnal cognitive arousal levels were positively associated with PSG sleep latency, which is consistent with prior reports.22–27 Moreover, we found that changes in nocturnal cognitive arousal were associated with changes in sleep latency such that decreases in nocturnal cognitive arousal were linked to falling asleep faster on PSG. To the contrary, nocturnal cognitive arousal was not associated with sleep maintenance difficulties. Self-reported somatic arousal at night was not associated with latency or maintenance parameters on PSG.
Although our data and prior studies9 indicate that standard CBTI and SRT may not therapeutically benefit objective wakefulness at night, identifying the connection between nocturnal cognitive arousal and objective sleep latency offers a potential entry point to improve patients’ ability to fall asleep. Standard CBTI does not emphasize focus on cognitive arousal such as rumination or worry, nor is cognitive arousal considered a key therapeutic target. Unsurprisingly, CBTI has not demonstrated strong or robust effects on the tendency to ruminate or worry,20,44 although even modest reductions in cognitive-emotional arousal facilitate treatment-response in CBTI.45 As experimental studies show that inducing rumination prolongs PSG and actigraphy-defined sleep latency,22–26 we may consider that therapeutically reducing nocturnal cognitive arousal with insomnia therapies augmented to better target these cognitive-emotional symptoms may have downstream effects on objective sleep. Future studies should determine whether enhancing CBTI (or other efficacious insomnia therapies) to defuse ruminative thought processes may improve objective sleep measures. Potential therapies and/or augmentation strategies to better improve cognitive-emotional arousal in insomnia may include cognitive therapy for insomnia,46 mindfulness-based therapy for insomnia,47 and even components of rumination-focused cognitive behavioral therapy48 or emotion regulation therapy.49
Limitations and future directions
The present study should be interpreted in light of certain limitations. Our primary limitation concerns limits of generalizability related to our patient population. As these patients reported insomnia that onset or was exacerbated by the menopause transition, results from our postmenopausal sample may not generalize to men or younger women. Further, etiological processes for menopausal insomnia may differ compared to the broader adult insomnia population. Indeed, levels of nocturnal cognitive arousal were lower in this sample than what has been reported by other insomnia patient populations, which may reflect differing etiology. Given the strong association between cognitive arousal and sleep latency, it is unsurprising then that PSG sleep latencies were largely within normal limits for this sample per quantitative criteria for objective findings.50 Thus, the nature of our sample limited our ability to detect potential CBTI and SRT treatment effects on PSG sleep latency due to restricted range. Relatedly, our study included entry criteria for PSG-based wake after sleep onset. While this is a strength for detecting improvements in objective sleep, this entry criterion limited may have contributed to our inability to testing other operationalizations of objective sleep disturbance by producing under-representation of patients with prolonged objective sleep latency and over-representation of those with objective sleep maintenance. Future studies testing treatment effects of CBTI and SRT should do so in more representative samples that allow testing different operationalizations of objective sleep disturbance, which may provide important evidence regarding clinical utility.
Importantly, in the present study, all subjects were required to stay in bed for 8 hours each night. Although this standardizes observation, it also creates a behavioral demand for maintaining sleep while being recorded, which itself could disrupt sleep. Along these lines, the pattern of wake after sleep onset may have been altered with CBTI or SRT. That is, if pretreatment sleep maintenance issues were widespread throughout the night, but then consolidated to early morning awakenings, this may thereby reflect consolidated sleep with early morning awakening possibly indicating that the patient does not need the full 8 hours of sleep allotted to them.
It is possible or even likely that sleep restriction (as a standalone treatment or as a component of CBTI) decreases objective sleep latency, particularly before complete titration of the sleep period. Even so, acute reductions in sleep latency are not necessarily therapeutic or beneficial if (1) pretreatment sleep latency is not excessive and (2) acute gains are not durable over the long-term. Notably, posttreatment PSG in the SRT was 2 weeks after treatment began, whereas PSG after CBTI was conducted after ~6 weeks of therapy. Therefore, it is possible that any acute sleep restriction-related changes in PSG sleep latency in the CBTI group were observable before titrating sleep schedules out. Research is needed to determine whether insomnia patients with prolonged objective sleep latency may experience a substantial and durable decrease in latency (on actigraphy or PSG) in response to sleep restriction (as standalone treatment or a component of CBTI) or whether acute movement on this index merely reflects acute sleep deprivation.
By extension, we previously reported that CBTI and SRT had no clinically meaningful effects on rumination and worry in this sample20 and, in the present study, we showed that these therapies largely did not improve objective sleep relative to control. Despite the lack of treatment effects, we observed that decreases in nocturnal cognitive arousal were associated with decreases in PSG sleep latency. But because these treatments are ill-designed to improve ruminative cognitive processes or objective sleep, the observed effect size in the present study may be underestimated. Further, it is possible that decreases in cognitive arousal may also be associated with other changes in objective sleep, but that a lack of treatment effects on cognitive arousal and objective sleep prevented our ability to detect such effects. Finally, the small sample size and PSG data loss may have limited our ability to detect effects by reducing statistical power. Even so, null findings presented here are consistent with the extant literature.
Conclusions
Postmenopausal insomnia patients with objective sleep disturbance appeared to have blunted treatment response to CBTI and SRT, whereas patients without objective sleep findings were more responsive to these insomnia therapies. In addition, cognitive and behavioral treatments for insomnia produced limited improvement in objective nocturnal wakefulness, yet alleviation of objective sleep disturbance was not necessary for insomnia remission based on patient-reported outcomes. It remains unclear, however, whether enhancing treatment effects on objective sleep could potentially improve outcomes for insomnia patients with objective sleep findings. Despite the lack of robust treatment effects, exploratory analyses suggest that reductions in nocturnal cognitive arousal are linked to reductions in objective sleep latency. This identifies cognitive arousal as a potential therapeutic target (and potential triaging variable) to facilitate improvements in objective sleep. Even so, therapeutic targets for psychological insomnia interventions to improve objective sleep maintenance remain elusive. Augmentation strategies using pharmacotherapy, possibly involving newer wake-inhibiting orexin antagonists that produce superior effects on objective total sleep time and sleep maintenance than sleep-promoting benzodiazepine receptor agonists,51,52 may enhance response to insomnia therapy in patients with objective sleep disturbance.
Supplementary Material
Highlights.
Objective sleep disturbance predicted poor response to insomnia therapy
Cognitive and behavioral insomnia treatments may not improve objective sleep
Broadly, treatment effects may not be necessary insomnia therapy success
Unclear whether improving objective sleep may enhance outcomes for certain patients
Improving nocturnal cognitive arousal may reduce objective sleep latency
Acknowledgements:
This study was funded by the National Institute of Nursing Research (R01 NR013959, PI: Drake). Dr. Cheng’s effort was supported by the National Heart, Lung, and Blood Institute (K23 HL138166, PI: Cheng).
Disclosure statement: Dr. Cheng has received research support from Harmony Biosciences. Dr. Roth. has received research support from Aventis, Cephalon, Glaxo Smith Kline, Neurocrine, Pfizer, Sanofi, Schering-Plough, Sepracor, Somaxon, Syrex, Takeda, TransOral, Wyeth and Xenoport and has acted as a consultant for Abbott, Acadia, Acoglix, Actelion, Alchemers, Alza, Ancil, Arena, Astra Zeneca, Aventis, AVER, BMS, BTG, Cephalon, Cypress, Dove, Elan, Eli Lilly, Evotec, Forest, Glaxo Smith Kline, Hypnion, Impax, Intec, Intra-Cellular, Jazz, Johnson & Johnson, King, Lundbeck, McNeil, Medici Nova, Merck & Co., Neurim, Neurocrine, Neurogen, Novartis, Orexo, Organon, Prestwick, Procter-Gamble, Pfizer, Purdue, Resteva, Roche, Sanofi, Schering-Plough, Sepracor, Servier, Shire, Somaxon, Syrex, Takeda, TransOral, Vanda, Vivometrics, Wyeth, Yamanuchi, and Xenoport. Dr. Drake has received research support from Merck & Co., Eisai Co., Jazz, and has served on speaker’s bureau for Harmony Biosciences. No other financial or non-financial interests exist.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Name: Behavioral Treatment of Menopausal Insomnia: Sleep and Daytime Outcomes
URL: clinicaltrials.gov
Registration: NCT01933295
References
- 1.Hirshkowitz M. Polysomnography and Beyond In: Kryger M, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th ed.: Elsevier; 2017:1564–1566. e1563. [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. Washington DC: American Psychiatric Association; 2013. [Google Scholar]
- 3.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 4.Reite M, Buysse D, Reynolds C, Mendelson W. The use of polysomnography in the evaluation of insomnia. Sleep. 1995;18(1):58–70. [DOI] [PubMed] [Google Scholar]
- 5.Littner M, Hirshkowitz M, Kramer M, et al. Practice parameters for using polysomnography to evaluate insomnia: an update. Sleep. 2003;26(6):754–760. [DOI] [PubMed] [Google Scholar]
- 6.Ong JC, Arnedt JT, Gehrman PR. Insomnia diagnosis, assessment, and evaluation In: Kryger M, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th ed. Philadelphia: Elsevier; 2017:785–793. e784. [Google Scholar]
- 7.Reynolds III CF, Kupfer DJ, Buysse D, Coble PA, Yeager A. Subtyping DSM-III-R primary insomnia: A literature review by the DSM-IV work group on sleep disorders. Am J Psychiatry. 1991;148(4):432–438. [DOI] [PubMed] [Google Scholar]
- 8.Edinger JD, Krystal AD. Subtyping primary insomnia: is sleep state misperception a distinct clinical entity? Sleep medicine reviews. 2003;7(3):203–214. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell L, Bisdounis L, Ballesio A, Omlin X, Kyle S. The impact of cognitive behavioural therapy for insomnia on objective sleep parameters: A meta-analysis and systematic review. Sleep Medicine Reviews. 2019. [DOI] [PubMed] [Google Scholar]
- 10.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep medicine reviews. 2013;17(4):241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez-Mendoza J The insomnia with short sleep duration phenotype: an update on it’s importance for health and prevention. Current opinion in psychiatry. 2017;30(1):56–63. [DOI] [PubMed] [Google Scholar]
- 12.Bathgate CJ, Edinger JD, Krystal AD. Insomnia patients with objective short sleep duration have a blunted response to cognitive behavioral therapy for insomnia. Sleep. 2017;40(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller CB, Espie CA, Bartlett DJ, Marshall NS, Gordon CJ, Grunstein RR. Acceptability, tolerability, and potential efficacy of cognitive behavioural therapy for Insomnia Disorder subtypes defined by polysomnography: A retrospective cohort study. Scientific reports. 2018;8(1):6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Annals of internal medicine. 2016;165(2):125–133. [DOI] [PubMed] [Google Scholar]
- 15.Lovato N, Lack L, Kennaway DJ. Comparing and contrasting therapeutic effects of cognitive-behavior therapy for older adults suffering from insomnia with short and long objective sleep duration. Sleep medicine. 2016;22:4–12. [DOI] [PubMed] [Google Scholar]
- 16.Rochefort A, Jarrin DC, Bélanger L, Ivers H, Morin CM. Insomnia treatment response as a function of objectively measured sleep duration. Sleep medicine. 2019;56:135–144. [DOI] [PubMed] [Google Scholar]
- 17.Crönlein T, Wetter TC, Rupprecht R, Spiegelhalder K. Cognitive behavioral treatment for insomnia is equally effective in insomnia patients with objective short and normal sleep duration. Sleep medicine. 2018. [DOI] [PubMed] [Google Scholar]
- 18.Dietch JR, Taylor DJ. The enigma of objective and subjective measurement of response to Cognitive Behavioral Therapy for Insomnia: Call to action. Sleep Medicine Reviews 2019. [DOI] [PubMed] [Google Scholar]
- 19.Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10(1):19–28. [DOI] [PubMed] [Google Scholar]
- 20.Kalmbach DA, Cheng P, Arnedt JT, et al. Treating Insomnia Improves Depression, Maladaptive Thinking, and Hyperarousal in Postmenopausal Women: Comparing Cognitive-Behavioral Therapy for Insomnia (CBTI), Sleep Restriction Therapy, and Sleep Hygiene Education. Sleep Medicine. 2019;55:124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalmbach DA, Cheng P, Arnedt JT, et al. Improving Daytime Functioning, Work Performance, and Quality of Life in Postmenopausal Women With Insomnia: Comparing Cognitive Behavioral Therapy for Insomnia, Sleep Restriction Therapy, and Sleep Hygiene Education. Journal of Clinical Sleep Medicine. 2019;15(07):999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall M, Buysse D, Reynolds C, Kupfer D, Baum A. Stress-related intrusive thoughts disrupt sleep onset and continuity. Sleep Research. 1996;25:163. [Google Scholar]
- 23.Wuyts J, De Valck E, Vandekerckhove M, et al. The influence of pre-sleep cognitive arousal on sleep onset processes. International Journal of Psychophysiology. 2012;83(1):8–15. [DOI] [PubMed] [Google Scholar]
- 24.Galbiati A, Giora E, Sarasso S, Zucconi M, Ferini-Strambi L. Repetitive thought is associated with both subjectively and objectively recorded polysomnographic indices of disrupted sleep in insomnia disorder. Sleep medicine. 2018;45:55–61. [DOI] [PubMed] [Google Scholar]
- 25.Gross RT, Borkovec T. Effects of a cognitive intrusion manipulation on the sleep-onset latency of good sleepers. Behavior Therapy. 1982;13(1):112–116. [Google Scholar]
- 26.Zoccola PM, Dickerson SS, Lam S. Rumination predicts longer sleep onset latency after an acute psychosocial stressor. Psychosomatic Medicine. 2009;71(7):771–775. [DOI] [PubMed] [Google Scholar]
- 27.Kalmbach DA, Buysse DJ, Cheng P, Roth T, Yang A, Drake CL. Nocturnal cognitive arousal is associated with objective sleep disturbance and indicators of physiologic hyperarousal in good sleepers and individuals with insomnia disorder. Sleep medicine. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stepanski EJ, Wyatt JK. Use of sleep hygiene in the treatment of insomnia. Sleep medicine reviews. 2003;7(3):215–225. [DOI] [PubMed] [Google Scholar]
- 29.Perlis ML, Jungquist C, Smith MT, Posner D. Cognitive behavioral treatment of insomnia: A session-by-session guide. Vol 1: Springer Science & Business Media; 2006. [Google Scholar]
- 30.Riemann D, Perlis ML. The treatments of chronic insomnia: a review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep medicine reviews. 2009;13(3):205–214. [DOI] [PubMed] [Google Scholar]
- 31.Miller CB, Espie CA, Epstein DR, et al. The evidence base of sleep restriction therapy for treating insomnia disorder. Sleep medicine reviews. 2014;18(5):415–424. [DOI] [PubMed] [Google Scholar]
- 32.Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus C, Vaughn BV. The AASM manual for the scoring of sleep and associated events. Rules, Terminology and Technical Specifications, Darien, Illinois, American Academy of Sleep Medicine. 2012;176. [Google Scholar]
- 33.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep medicine. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 34.Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicassio PM, Mendlowitz DR, Fussell JJ, Petras L. The phenomenology of the pre-sleep state: the development of the pre-sleep arousal scale. Behaviour research and therapy. 1985;23(3):263–271. [DOI] [PubMed] [Google Scholar]
- 36.Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2018;42(2):zsy217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bathgate CJ, Edinger JD, Wyatt JK, Krystal AD. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016;39(5):1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertisch SM, Pollock BD, Mittleman MA, et al. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: Sleep Heart Health Study. Sleep. 2018;41(6):zsy047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johann AF, Hertenstein E, Kyle SD, et al. Insomnia with objective short sleep duration is associated with longer duration of insomnia in the Freiburg Insomnia Cohort compared to insomnia with normal sleep duration, but not with hypertension. PloS one. 2017;12(7):e0180339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maglione JE, Ancoli-Israel S, Peters KW, et al. Depressive symptoms and subjective and objective sleep in community-dwelling older women. Journal of the American Geriatrics Society. 2012;60(4):635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maglione JE, Ancoli-Israel S, Peters KW, et al. Subjective and objective sleep disturbance and longitudinal risk of depression in a cohort of older women. Sleep. 2014;37(7):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best practice & research Clinical endocrinology & metabolism. 2010;24(5):731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yap Y, Slavish DC, Taylor DJ, Bei B, Wiley JF. Bi-directional Relations between Stress and Self-Reported and Actigraphy-Assessed Sleep: A Daily Intensive Longitudinal Study. Sleep. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalmbach DA, Cheng P, O’Brien LM, et al. A randomized controlled trial of digital cognitive behavioral therapy for insomnia in pregnant women. Sleep Medicine. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng P, Kalmbach DA, Cuamatzi-Castelan A, Muragan N, Drake CL. Depression prevention in digital cognitive behaviroal therapy for insomnia: Is rumination a mediator? Journal of Affective Disorders. 2020. [DOI] [PubMed] [Google Scholar]
- 46.Harvey AG. A cognitive theory and therapy for chronic insomnia. Journal of Cognitive Psychotherapy. 2005;19(1):41–59. [Google Scholar]
- 47.Ong JC. Mindfulness-based therapy for insomnia. Washington DC: American Psychological Association; 2017. [Google Scholar]
- 48.Watkins ER. Rumination-focused cognitive-behavioral therapy for depression. New York: The Guilford Press; 2018. [Google Scholar]
- 49.Renna ME, Quintero JM, Fresco DM, Mennin DS. Emotion regulation therapy: a mechanism-targeted treatment for disorders of distress. Frontiers in psychology. 2017;8:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lichstein K, Durrence H, Taylor D, Bush A, Riedel B. Quantitative criteria for insomnia. Behaviour research and therapy. 2003;41(4):427–445. [DOI] [PubMed] [Google Scholar]
- 51.Zammit G, Mayleben D, Kumar D, Murphy P, Moline M. EFFICACY OF LEMBOREXANT COMPARED WITH ZOLPIDEM EXTENDED RELEASE AND PLACEBO IN ELDERLY SUBJECTS WITH INSOMNIA: RESULTS FROM A PHASE 3 STUDY (SUNRISE 1). The American Journal of Geriatric Psychiatry. 2019;27(3):S154–S155. [Google Scholar]
- 52.Struyk A, Gargano C, Drexel M, et al. Pharmacodynamic effects of suvorexant and zolpidem on EEG during sleep in healthy subjects. European Neuropsychopharmacology. 2016;26(10):1649–1656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


