Abstract
Study Objectives
Previous studies were inconsistent with regard to the association of sleep dysfunction on the brain’s gray matter volume (GMV). The current study set out to investigate if there is a moderating effect of sex on the relationship between sleep quality in healthy individuals and GMV.
Methods
We applied voxel-based morphometry in 1,074 young adults of the “Human Connectome Project.” An analysis of variance with the factors “sleep quality” (good/poor according to the Pittsburgh Sleep Quality Index, cutoff >5) and “sex” (male, female) on GMV was conducted. Additionally, linear relationships between sleep quality and GMV were tested.
Results
The analysis of variance yielded no main effect for sleep quality, but an interaction between sex and sleep quality for the right superior frontal gyrus. Post hoc t-tests showed that female good sleepers in comparison to female poor sleepers had larger GMV in the right parahippocampal gyrus extending to the right hippocampus (whole-brain family-wise error [FWE]-corrected), as well as smaller GMV in the right inferior parietal lobule (whole-brain FWE-corrected) and the right inferior temporal gyrus (whole brain FWE-corrected). There were no significant effects when comparing male good sleepers to male poor sleepers. Linear regression analyses corroborated smaller GMV in the right parahippocampal gyrus in women with poor sleep quality.
Conclusions
Poor sleep quality was associated with altered GMV in females, but not in males. Future studies are needed to investigate the neurobiological mechanisms that underlie the sex differences in the association of sleep quality and brain differences found in this study.
Keywords: gray matter volume, PSQI, sex, sleep quality
Statement of Significance.
The present study is the first study investigating sex differences with regard to the association of sleep dysfunction and brain structure. In a well-powered sample of healthy individuals, it demonstrated that sex moderated the association of gray matter volume and sleep quality. These results may stimulate future studies of sleep dysfunction and associated brain mechanisms to consider “sex” as a possible moderator variable.
Introduction
Sleep quality is a broad concept [1] describing somebody’s self-reported sleep experience that can vary in a continuum from very good to extremely poor. While sleep disorders, such as insomnia, sleep-related breathing, or movement disorder, are diagnosed according to the criteria of the International Classification of Sleep Disorders [2], sleep quality can be assessed in the nonclinical population by means of psychometric questionnaires [3]. The most widely used questionnaire is the Pittsburgh Sleep Quality Inventory (PSQI) [4] that incorporates diagnostic criteria of the evaluation of clinical sleep dysfunction. A total score beyond a cutoff value of 5 indicates poor sleep quality, which may point to a clinically relevant sleep disorder. The PSQI is highly correlated to other measures of sleep quality [3], e.g. to the insomnia severity index in a community sample (r = 0.80) [5].
Poor sleep quality is widespread in the general population with a prevalence of 8%−18% of people being dissatisfied with their sleep quality or quantity [6]. Possible causes are of physiological (e.g. hormonal status and overactivity of the arousal system), psychological (e.g. stressful life events), and social (e.g. socioeconomic risk factors) nature [7]. It is a well-replicated finding that there are differences in sleep architecture and sleep quality between men and women. Although women have higher sleep efficiency, a lower percentage of stage 1 sleep, a higher percentage of slow-wave sleep, and longer rapid eye movement (REM) latency than men [8], they report lower self-reported sleep quality with more sleep disturbances and insomnia symptoms compared to men [9, 10]. To date the reasons for this discrepancy are not clear. Possibly objective polysomnographic data are too crude to capture the subtle physiologic variations responsible for self-reported poor sleep quality in women [11]. Sleep disturbances in women in comparison to men start with the beginning of menses [12, 13] and continue across lifespan especially during times of hormonal change (pregnancy, postpartum, and menopause) [14, 15]. In women, but not in men, self-reported sleep quality declines with age, with older women having more trouble with waking up during the night and waking up too early [11]. Risk ratios for women developing insomnia increase with age from 1.28 in young adults to 1.73 in the elderly [16]. These sex differences persist even after controlling for chronic health conditions [11] and psychiatric disorders with a female preponderance [16, 17], such as depression and anxiety, which are highly correlated with sleep disturbances [9, 18, 19]. Poor sleep quality is also related to pain and somatic symptoms [20], as well as stress indicators [21], especially in women.
Only a few studies have examined the association of self-reported sleep and brain structure in healthy individuals. Investigating sleep quality in community-dwelling adults between 20 and 84 years, a cross-sectional analysis showed an association between poor sleep quality and smaller gray matter brain volume (GMV) in the right superior frontal cortex [22], which remained significant after removing older participants. Another study found no cross-sectional relationship between self-reported sleep measures and hippocampal volume using data from different large data banks [23]. In smaller, clinical samples of patients with insomnia, studies of structural brain changes going along with sleep dysfunction yielded mixed findings. Investigating chronic insomnia in middle-aged to elderly participants with voxel-based morphometry (VBM), smaller GMV has been found in the left orbitofrontal cortex, the bilateral anterior and posterior precuneus [24] as well as the hippocampus [25, 26]. On the other hand, larger GMV has been detected in younger patients with chronic insomnia in the rostral anterior cingulate cortex volume [27] and in the left orbitofrontal cortex, the right rostral anterior cingulate cortex, bilateral rostral middle frontal gyrus, and right fusiform area [28]. Both of these studies used surface-based morphometry in younger samples (<40 years), though, which might point to different neuropathological mechanisms in older and younger populations and/or different results due to methodological reasons. In a comprehensive and well-controlled study using FreeSurfer-based analyses as well as VBM, Spiegelhalder et al. [29] did not find any gray or white matter differences in patients with insomnia (age 18–67 years) compared to matched controls. Likewise, Winkelman et al. [30] could not replicate hippocampal volume differences in primary insomnia. A recent activation-likelihood estimation meta-analysis study combining functional and structural imaging results did not find consistent patterns of abnormal brain alterations in insomnia disorder [31].
Except for one study [30], none of the aforementioned studies investigated the sex differences in the association of GMV with sleep dysfunction, either by grouping together male and female participants or by partialling out all potential sex-related influences. Winkelman et al. [30] tested for associations between insomnia, sex, and hippocampal volume, but results did not reach significance, possibly due to the small sample size.
The current study set out to investigate the moderating effect of sex on the relationship between sleep quality and the brain’s gray matter. To avoid shortcomings of previous studies in clinical samples (small sample sizes, a lacking or too liberal statistical control for multiple comparisons and confounders), we investigated the population-based sample of the Human Connectome Project (HCP; Courtesy of the Laboratory of Neuro Imaging and Martinos Center for Biomedical Imaging, Consortium of the Human Connectome Project—www.humanconnectomeproject.org). In the HCP Young Healthy Adults Project, participants with a significant history of psychiatric disorder, substance abuse, and neurological or cardiovascular disease are excluded, enabling the study of poor sleep quality and GMV while considerably reducing confounding influences. Since a sex effect has been consistently reported for the self-reported perception of sleep quality [7], there may be sex differences with regard to the association of sleep quality and brain structure. We investigated this issue in a well-powered sample without prior hypotheses (p < 0.05; family-wise error [FWE] correction over the whole brain volume). Additionally, we investigated GMV in regions of interest in brain areas that have been associated with sleep dysfunction in previous studies (for a summary, see Ref. [32]). Because of the prominent role of sleep in hippocampal functioning [33] and inconsistent previous results [23, 25, 26, 30], we were particularly interested in the association of sleep quality and hippocampal GMV. We hypothesized smaller hippocampal GMV in participants with poor sleep quality.
Methods
Participants
Data used in the preparation of this work were obtained from the MGH-USC HCP database (https://ida.loni.usc.edu/login.jsp). The HCP (principal investigators: Bruce Rosen, MD, PhD, Martinos Center at Massachusetts General Hospital; Arthur W. Toga, PhD, University of Southern California; Van J. Weeden, MD, Martinos Center at Massachusetts General Hospital) is supported by the National Institute of Dental and Craniofacial Research, the National Institute of Mental Health, and the National Institute of Neurological Disorders and Stroke. Collectively, the HCP is the result of efforts of co-investigators from the University of Southern California, Martinos Center for Biomedical Imaging at Massachusetts General Hospital, Washington University, and the University of Minnesota. The “Young Healthy Adults Project” comprises imaging, behavioral, and genetic datasets of 1,200 participants in the age range of 22–35 years. Exclusion criteria are severe neurodevelopmental disorders (e.g. autism), documented neuropsychiatric disorders (e.g. schizophrenia or depression), or neurologic disorders (e.g. Parkinson’s disease), as well as diabetes or high blood pressure [31]. For the current study, we included all participants who underwent a T1 brain scan and filled out the Pittsburgh Sleep Quality Index (PSQI, see the Questionnaires section) [4] (n = 1,113). To rule out a confounding influence of enhanced levels of depression, an exclusion criterion was a score of at least 70 (sex and age-adjusted t-score, two standard deviations above the mean) or missing value on the Adult Self Report (ASR) Questionnaire, Scale “Depressive Problems” [34, 35] (n = 37) (see the Questionnaires section). Because we included the variable “education” as a covariate in our VBM analysis, participants with a missing value for “education” (n = 2) were also excluded leaving 1,074 participants for final analysis. Of those, 489 (45.5%) were male, 585 (55.5%) female (mean age 28.8 years, SD 3.7).
Questionnaires
For the current study, the following data were used from the HCP database:
The PSQI [4] is a self-rated questionnaire, which assesses sleep quality and disturbances over a 1-month interval. Its minimum score is 0, its maximum score 21. A total of not greater than 5 indicates good sleep quality, a total of greater than 5 poor sleep quality. Years of education completed were coded as <11 = 11; 12; 13; 14; 15; 16; 17+ = 17. Handedness was assessed with the Edinburgh Handedness Inventory [36].
Negative numbers mean that a person is more left-handed than right-handed, while positive numbers mean that a person is more right-handed than left-handed. Total household income was coded as <$10,000 = 1; 10,000–19,999 = 2; 20,000–29,999 = 3; 30,000–39,999 = 4; 40,000–49,999 = 5; 50,000–74,999 = 6; 75,000–99,999 = 7; ≥100,000 = 8. The body mass index (BMI) was measured as 703 × (weight (lb)/height (in.)2). Systolic and diastolic blood pressure were indicated to characterize the sample. The perceived stress survey (NIH Toolbox Perceived Stress Fixed Form Age 18+) is a self-report measure that accesses how unpredictable, uncontrollable, and overloaded respondents find their lives. The Unadjusted Scale Score has a mean of 50, SD of 10. Higher scores are indicative of more perceived stress. From the Achenbach ASR, Syndrome Scales and DSM-Oriented Scale Scale I: Depressive Problems (sex and age-adjusted t-score) and Scales I–III: Internalizing Problems (ASR Anxious/Depressed: scale I, ASR Withdrawn: scale II, ASR Somatic Complaints: scale III, sex and age-adjusted t-score) were used. The Pain intensity raw score consists of a single item measuring immediate (i.e. acute) pain in adults. It asks a participant to rate the level of pain experienced over the last 7 days on a 0–10 scale, with 0 representing no pain and 10 representing the worst imaginable pain on this measure.
Image acquisition
MR images were obtained using a 3-T Siemens MR imaging scanner (Connectome Skyra; Siemens Medical Systems, Erlangen, Germany) equipped with a 32-channel head coil using a T1-weighted magnetization-prepared rapid acquisition gradient-echo sequence and the following parameters: matrix size: 260 × 311, voxel size: 0.7 mm isotropic, repetition time = 2,400 ms, echo time = 2.14 ms, flip angle =8°. For an in-depth description of the sequence used see the Reference Manual of the HCP (WU-Minn HCP 1,200 Subjects Release: Reference Manual –>Appendix I—Protocol Guidance and HCP Session Protocols).
Preprocessing of MRI data
Statistical Parametric Mapping 12 (SPM12; Wellcome Department of Cognitive Neurology, University of London, London, UK) and the Computational Anatomy Toolbox (CAT12; Christian Gaser, Department of Psychiatry, University of Jena, Jena, Germany) running on MATLAB (MathWorks, Natick, MA) were used to preprocess the structural images with default parameters given by the CAT12 toolbox. The preprocessing steps involved segmentation into GMV, white matter, and cerebrospinal fluid; affine registration to the stereotactic Montreal Neurological Institute (MNI) space; high-dimensional DARTEL normalization; and nonlinear modulation using the Jacobian determinants derived from the normalization process. We smoothed the modulated GMV images using an 8-mm full width at half maximum Gaussian kernel to compensate for potential inaccuracies during the normalization step and to render the intensities more normally. An image quality rating was calculated automatically by the CAT12 toolbox segmentation pipeline as well as the total intracranial volume (TIV) used later as a nuisance variable.
Statistical analyses
Group comparisons of individuals with good and poor sleep quality were performed with SPM12 (Wellcome Department of Imaging Neuroscience) by applying a 2 × 2 analysis of variance design with the factors “sleep quality” (good/ poor) and “sex” (male/ female). Using F-contrasts, the main effect of sleep quality (good sleepers versus poor sleepers) as well as the interaction “sleep quality by sex” was tested. Significant F-tests were followed by post hoc t-tests including the positive (for good sleepers > poor sleepers and males > females) and negative interactions (for good sleepers > poor sleepers and females > males) of sleep quality by sex, as well as the contrasts, “male good sleepers > male poor sleepers,” “male poor sleepers > male good sleepers,” and “female good sleepers > female poor sleepers,” “female poor sleepers > female good sleepers.” Additionally, multiple regression analyses were performed with SPM12 to test linear relationships between sleep quality and regional GMV.
We entered the variables “years of education” and “age” as covariates to consider their correlation with TIV. BMI, handedness, and internalizing problems were added as further covariates to account for group differences between males and females and/or good and poor sleepers in these variables. The image quality rating and TIV were included as nuisance variables during statistical analysis to remove the related variance. For thresholding, we applied p < 0.05, FWE-corrected for multiple comparisons for the whole brain. We additionally applied a region-of-interest (ROI) analysis (p < 0.05, FEW-corrected for multiple comparisons within ROIs, see below) using a composite anatomical mask including brain areas that were involved in sleep dysfunction according to previous neuroimaging studies. Spatial assignment of GMV differences above the statistical threshold was conducted with the SPM Anatomy Toolbox Version 2.2c [37–39].
Based on previous literature (see Ref. [32] and the respective references at the brain areas listed below), an ROI analysis was performed using a composite mask that included the following brain areas (p < 0.05, FWE-corrected for multiple comparisons within ROI):
Superior parietal lobule, BA 7 [24]: The mask provided in Anatomy included the areas 7A, 7M, 7P, 7PC [40].
Prefrontal cortex [22, 28]: Masks provided in Anatomy included the frontal pole (areas Fp1 and Fp2) [41] and the orbitofrontal cortex (areas OFC 1, OFC 2, OFC 3) [42].
Rostral anterior cingulum [27, 28]: Mask included s32 provided in Anatomy [43].
Left N. Caudatus [44]: Mask “Caudate_left” from AAL [45].
Hippocampus [25, 26]: Mask provided in Anatomy including CA1–CA3, dentate gyrus, Subiculum, HATA region [46].
Amygdala [47]: Mask provided in Anatomy including AStr, CM, LB, SF [46].
Results
Demographic characterization
The mean PSQI score of our sample was 4.71 (SD = 2.67, range: 0–16). About 330 participants (30.7%) had a PSQI score of greater than 5 that is associated with poor sleep quality [4]. Women had a higher PSQI score (M = 4.85, SD = 2.8) than men (M = 4.54, SD = 2.5) (t1,071 = −1.9, p = 0.028, one-tailed) reflecting worse sleep quality in women. Women (M = 29.6, SD = 3.6) were older than men (M = 27.9, SD = 3.6) (t1,072 = −7.4, p = 0.001), more right-handed (M = 71.2, SD = 42.9) in comparison to men (M = 61.2, SD = 43.3) (t1,072 = −3.8, p = 0.001), and had less internalizing problems (M = 46.3, SD = 11.2) than men (M = 48.2, SD = 10.4) (t1,072 = 2.9, p = 0.004). Men had higher systolic (M = 128.3, SD = 13.1) and diastolic (M = 78.4, SD = 10.6) blood pressure than women (systolic: M = 119.7, SD = 13.7; diastolic: M = 74.9, SD = 10.5) (systolic: t1,059 = 10.4, p = 0.001; diastolic: t1,059 = 5.4, p = 0.001). There were no differences between men and women with regard to BMI, education, perceived stress, or pain. For differences between male and female good and poor sleepers, please refer to Table 1.
Table 1.
Characterization of the Sample
| Males | Females | |||||
|---|---|---|---|---|---|---|
| PSQI ≤5 | PSQI >5 | PSQI ≤5 | PSQI >5 | |||
| Number | 346 (70.8%) | 143 (29.2%) | 398 (68%) | 187 (32%) | ||
| Mean (SD) | Mean (SD) | |||||
| PSQI | 3.27 (1.3) | 7.62 (1.7) | t 213 = −27.2*** | 3.28 (1.3) | 8.18 (2.2) | t 252 = −28.0*** |
| Age | 27.9 (3.7) | 27.9 (3.4) | n.s. | 29.6 (3.6) | 29.5 (3.5) | n.s. |
| Education | 14.9 (1.7) | 14.5 (1.9) | t 241 = 2.3* | 15.2 (1.7) | 14.6 (1.9) | t 332 = 3.2** |
| Handedness | 62.6 (43) | 57.8 (45) | n.s. | 74.0 (40) | 65.6 (47) | t 322 = 2.1* |
| Income | 5.2 (2.2) | 4.6 (2.1) | t 485 = 3.0** | 5.3 (2.1) | 4.8 (2.2) | t 580 = 2.5* |
| BMI | 26.8 (4.4) | 27.0 (4.3) | n.s. | 25.8 (5.5) | 27.5 (6.1) | t 330 = −3.2*** |
| BP systolic | 128.4 (13.6) | 128.0 (12.0) | n.s. | 118.9 (13.2) | 121.4 (14.8) | t 575 = −2.0* |
| BP diastolic | 78.3 (10.7) | 78.7 (10.2) | n.s. | 74.3 (9.9) | 76.4 (11.5) | t 314 = −2.2* |
| Perceived stress | 46.3 (8.6) | 50.0 (9.2) | t 487 = −4.2*** | 47.2 (8.2) | 50.7 (9.0) | t 582 = −4.7*** |
| ASR internalizing problems (T) | 46.7 (10.1) | 52.0 (10.3) | t 487 = −5.3*** | 44.8 (11.0) | 49.7 (11.0) | t 583 = −5.0*** |
| Pain | 1.17 (1.4) | 1.8 (1.9) | t 204 = −3.6*** | 1.14 (1.5) | 1.95 (2.3) | t 267 = −4.4*** |
n.s., not significant.
*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
VBM data
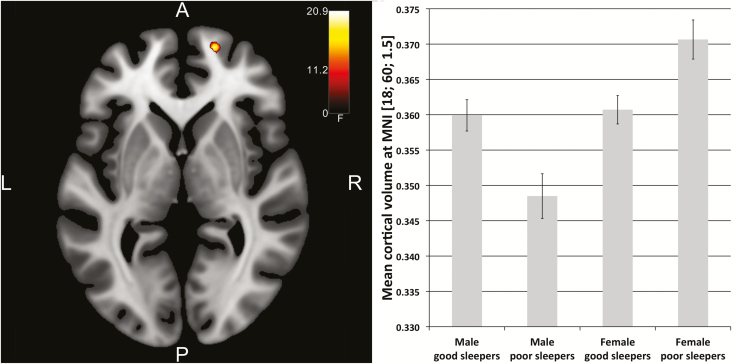
The analysis of variance showed a main effect for the factor “sex” (results are not reported here), but not for “sleep quality” (neither in the whole brain nor in the ROI approach. The left postcentral gyrus approached significance through MNI: −38, −27, 54, F = 23.57, p = 0.06, whole-brain FWE-corrected). The interaction of sex by sleep quality was significant in the right superior frontal gyrus (area Fp1) for the ROI approach (MNI: 18, 60, 2, F = 20.89, pFWE = 0.021, FWE-corrected for ROI). A post hoc t-test indicated that the positive interaction was significant in the right superior frontal gyrus (t = 4.57, p = 0.011, FWE-corrected for ROI) with male good sleepers showing larger GMV than male poor sleepers, but female good sleepers showing smaller GMV than female poor sleepers (Figure 1). The negative interaction showed a cluster in the right thalamus/hippocampus region (MNI: 26, −22, −8; 22, −32, −6, t = 3.63, p < 0.01 uncorrected) with female poor sleepers showing smaller GMV than the other three groups; however, this result did not survive the FWE correction for multiple testing.
Figure 1.
Left: Interaction of sleep quality (good/poor) and sex (male/female) in the right superior frontal gyrus (red hot colored area, axial slice plane MNI z = 1.5). For visualization purposes, a voxel-threshold of p < 0.001 (uncorrected) was used. SPM F-maps were thresholded with F ≥ 11.2. Right: Interaction effect as plotted for the four groups of male and female good and poor sleepers. Whereas male poor sleepers showed smaller GMV than male good sleepers, female poor sleepers showed larger GMV than female good sleepers in the right superior frontal gyrus.
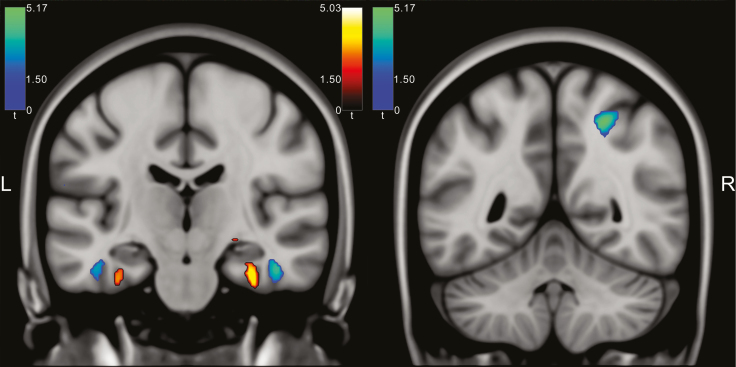
Comparing male good sleepers to male poor sleepers, there were no significant GMV differences neither for the whole brain nor for the ROI approach. Female good sleepers in comparison to female poor sleepers showed larger GMV in the right parahippocampal gyrus extending to the right hippocampus (MNI: 34, −16, −30, t = 5.03, p = 0.014, whole-brain FWE-corrected) and smaller GMV in the right inferior parietal lobule (MNI: 26, −51, 50, t = 5.17, p = 0.007, whole-brain FWE-corrected) and the right inferior temporal gyrus (MNI: 45, −15, −27, t = 4.76, p = 0.046, whole-brain FWE-corrected) (Figure 2; Table 2). Small volume correction did not add any significant areas.
Figure 2.
Left: Female good sleepers showed larger GMV in the right hippocampus/parahippocampal gyrus compared to female poor sleepers (red hot colored area) and smaller GMV in the right inferior temporal gyrus (blue-green colored area, coronal slice plane MNI y = −19). Right: Female good sleepers showed smaller GMV in the right inferior parietal lobule (blue-green colored area, coronal slice plane MNI y = −51). For visualization purposes, a voxel-threshold of p < 0.001 (uncorrected) was used. SPM t-maps were thresholded with t ≥1.5. Left-lateralized areas did not reach statistical thresholds corrected for multiple testing.
Table 2.
Analysis of Variance With the Factors “Sleep Quality” (Good, Poor) and “Sex” (Male, Female) on GMV in Good (n = 744) and Poor (PSQI >5, n = 330) Male and Female Sleepers and Subsequent Significant Post Hoc t-Tests
| Contrast | FWE correction | Side | MNI coordinates | p FWE | |||
|---|---|---|---|---|---|---|---|
| Main effect sleep quality | x | y | Z | F | |||
| Postcentral gyrus | Whole brain | L | −38 | −27 | 54 | 23.57 | 0.06 |
| Interaction sex by sleep quality | |||||||
| Superior frontal gyrus | ROI | R | 18 | 60 | 2 | 20.89 | 0.021 |
| Female good sleepers > female poor sleepers | t | ||||||
| Parahippocampal gyrus/hippocampus | Whole brain | R | 34 | −16 | −30 | 5.03 | 0.014 |
| Female poor sleepers > female good sleepers | t | ||||||
| Inferior parietal lobule | Whole brain | R | 26 | −51 | 50 | 5.17 | 0.007 |
| Inferior temporal gyrus | Whole brain | R | 45 | −15 | −27 | 4.76 | 0.046 |
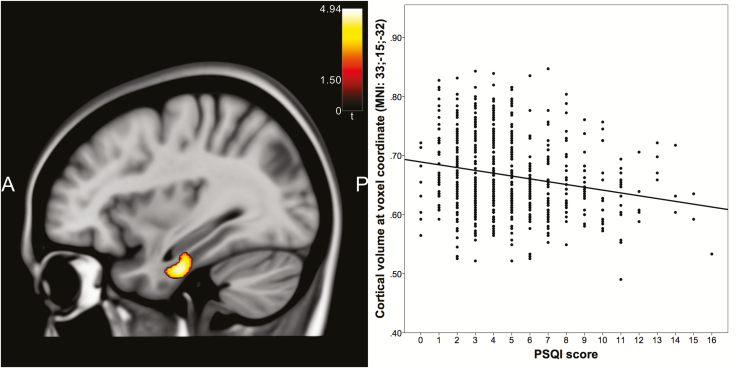
A regression analysis across all participants with PSQI values as independent and GMV as dependent variables revealed a trend toward significance in the right parahippocampal gyrus (MNI: 33, −16, −28, F = 18.50, p = 0.06, FWE-corrected for ROI) that became significant in the t-contrast showing that poor sleep quality went along with less GMV (MNI: 33, −16, −32, t = 4.30, p = 0.031, FWE-corrected for ROI). This effect was, however, only present in women (MNI: 33, −15, −32, t = 4.94, p = 0.023, whole-brain FWE-corrected, Figure 3), whereas in men there were no significant linear relationships between sleep quality and GMV in any brain area, not even after small-volume correction with the Hippocampus mask alone. The inverse relationship of female good sleepers showing smaller GMV was visible when using a more liberal statistical approach (uncorrected voxel threshold of p < 0.001, right inferior temporal gyrus: MNI: 45, −14, −30, t = 4.14; right inferior parietal lobule: MNI: 28, −52, 51, t = 3.74), but did not reach significance when correcting for multiple testing.
Figure 3.
A linear relationship between sleep quality and GMV in women in the right parahippocampal gyrus (sagittal slice plane MNI x = 33).
Discussion
The current study sought to investigate sex differences in the association of GMV and poor sleep quality that was measured with the PSQI. Previous studies were inconsistent with regard to the existence and direction (larger or smaller) of possible GMV differences associated with sleep dysfunction. We hypothesized that apart from the influences of confounders and small sample sizes, a moderating effect of sex may underlie these contradicting findings. We analyzed HCP0 data [49] that were collected from a population of young healthy adults (n = 1,074; mean age 28.8 years). A full factorial model with the factors sex and sleep quality yielded no main effect for sleep quality, but an interaction between sex and sleep quality for the right superior frontal gyrus, which showed larger GMV in male good sleepers compared to male poor sleepers, whereas in females this effect was inverse. Comparing female good sleepers to female poor sleepers, larger GMV was found in the right parahippocampal gyrus extending to the right hippocampus, as well as smaller GMV in the right inferior parietal lobule and the right inferior temporal gyrus. There were no significant effects when comparing male good sleepers to male poor sleepers. Linear regression analyses corroborated smaller GMV in the right parahippocampal gyrus in women with poor sleep quality. Our results therefore confirm an association of sleep dysfunction and altered GMV in accordance with previous studies [22, 24–28] (but see Refs [29, 30]). It further supports our model that sex has a moderating effect on the location and the kind of GMV differences associated with poor sleep quality.
Interaction of sleep quality and sex in the right superior frontal gyrus
Our hypothesis of a moderating effect of sex with regard to the association of sleep quality and GMV was significant in the right superior frontal gyrus that showed larger GMV in male good compared to poor sleepers, but smaller GMV in female good compared to poor sleepers. Despite this significant interaction, the effects within the groups of male and female sleepers were small. Our findings corroborate a previous population-based study [22] that found an association of poor sleep quality with smaller GMV exclusively in the right superior frontal gyrus. The results of the current study in a larger sample indicated that different mechanisms may be effective in men and women, which are associated with smaller, larger, or unchanged GMV in connection with poor sleep quality. In this context, it is important to note that the current study was cross-sectional and not designed to inform about the causal direction of an association between sleep quality and GMV alterations. In accordance with the notion of sleep serving a restorative function [50], the assumption that sleep quality influences brain structure is highly plausible but not exclusive. Although we investigated a young, healthy adult group without brain lesions, it cannot be completely ruled out that interindividual differences in brain structure affected self-reported sleep quality. Particularly with regard to the moderating effect of sex, it is also possible that there may be sex-specific factors that lead independently to both brain changes and changes in self-reported sleep quality. On the other hand, the advantage of having a young healthy sample is that confounders related to GMV alterations due to aging or somatic or psychiatric comorbidities are largely excluded. Additionally, we controlled for variables, which are known to be related to sex, such as TIV and internalizing problems including anxiety, depression, somatic complaints, and withdrawal. Another concern may be that the accuracy of the self-reported assessments could be impacted by sex, since men tend to be less reliable in their estimates of health issues, even if these sex differences are small [51]. Bearing in mind all these shortcomings, in the following, we will discuss our results in relation to our model that poor sleep quality causes GMV changes and that sex influences how sleep impacts the brain.
Smaller hippocampal GMV in female poor sleepers
In women, we found a linear relationship of poor sleep quality and smaller GMV in the right parahippocampal gyrus including the right hippocampus that was absent in men. These differences were rather small, so that the interaction of sleep quality by sex in this region became only significant when using a more liberal statistical threshold. Some previous clinical studies found smaller hippocampal GMV in patients with chronic insomnia [25, 26], but others did not [29, 30]. In addition to methodological differences of these studies including more or less liberal statistical thresholds, the Joo et al. study [25] investigated a sample consisting of 93% females that may have contributed to the positive results. We can only speculate about why in the current study hippocampal GMV was not associated with poor sleep quality in men. Sex differences in circadian timing systems including sleep-promoting circuits have been observed at many different levels of analysis, including genes, cells, tissues, and whole organisms, all of which can influence brain networks and behavior (for reviews see Refs [52, 53]). One important factor influencing sleep in men and women differently is sex-specific hormones that have receptors in sleep-regulating systems [15, 52]. Poor sleep quality in women is associated with low levels of estrogen and progesterone that prevail in the late luteal phase of the menstrual cycle and the first days of menstruation [54, 55], whereas the male sleep-wake circuitry is less responsive to sex steroids [15]. Using animal models, previous studies demonstrated that sleep fragmentation or deprivation leads to impairment in hippocampal-dependent memory as well as changes in cellular and molecular correlates involved in membrane excitability and synaptic plasticity within the hippocampus [56, 57]. A recent study in young adults found a correlation between sleep quality and their brain’s right hippocampal mean diffusivity indicating greater tissue density [58]. In accordance with the model that worse sleep quality in women compared to men in the current study resulted in smaller GMV in hippocampal and parahippocampal areas, in women, there may be an increased risk for impaired cognitive functioning, especially memory consolidation [59], and later dementia [60].
Larger GMV in the right inferior parietal lobule and right inferior temporal gyrus in female poor sleepers
Female poor sleepers had larger GMV in the right inferior parietal lobule and the right inferior temporal gyrus compared to female good sleepers. Previous studies investigating patients with insomnia reported larger GMV in the rostral anterior cingulate cortex volume [27, 28]. A larger volume of the anterior cingulate cortex was assumed to reflect increased activity, which was interpreted in the framework of the hyperarousal model of insomnia [61]. Larger GMV has been also reported for the left orbitofrontal cortex, bilateral rostral middle frontal gyrus, and right fusiform area [28] with the latter localized very close to the right inferior temporal gyrus that exhibited larger GMV in the current study. Larger GMV is usually reported in connection with motor [62] or cognitive [63, 64] training, although the opposite effect of smaller GMV in experts has also been found [65]. A recent review characterized the inferior parietal lobule together with the temporoparietal junction as a major network hub involved in multiple functional domains, such as attentional reorienting, self-perception, undirected thinking, memory, and social cognition [66]. Since there is a right dominance of attentional functions, we speculate that overactivity of the ventral attention network may be associated with micro-arousals during sleep, especially REM sleep [67], and may lead to larger GMV in right inferior parietal and temporal areas in female poor sleepers. However, future studies will have to investigate this issue in more detail.
Implications
The present study is the first investigation of sex moderating the association of sleep quality and brain structure and as such exploratory in nature. Our findings suggest that the association between sleep quality and GMV may be different in women than it is in men. Further studies have to corroborate these results and investigate their validity for different age groups and clinical conditions. Studies exploring the interdependencies of sleep quality, brain structure or function, and psychiatric conditions, such as depression or anxiety [68], should consider sex as a possible moderator variable, particularly in the light of sex disparities in all three domains. This would be particularly important when considering interventions that directly target abnormal brain functioning, such as repetitive transcranial magnetic stimulation for reducing depressive symptoms and improving sleep quality [69].
Limitations
The present study has some limitations that have to be mentioned: First the available sleep-related data were restricted to the PSQI [4], so there were no other questionnaires, polysomnography, or actigraphy data. Nevertheless, the PSQI is a widely used questionnaire with good psychometric properties that appropriately measures sleep quality in the nonclinical population [70]. Second, the current investigation was limited to the group of young, healthy adults and may have restricted generalizability. Poor sleep quality is a heterogeneous construct that includes all different kinds of etiologies for whom distinct neurobiological mechanisms may apply. These have to be investigated in further studies. On the other hand, because clinical sleep dysfunction incorporates aspects of poor sleep quality in the nonclinical population, neurobiological mechanisms derived from the current study also add to the understanding of clinical sleep dysfunction. Finally, as described in detail above, due to the cross-sectional design of this study, causal relationships between sleep dysfunction and altered GMV cannot be derived.
Summary
The current study set out to investigate the moderating effect of sex on the relationship between sleep quality and brain structure. In female good sleepers compared to poor sleepers, larger GMV was found in the right parahippocampal gyrus extending to the right hippocampus, as well as smaller GMV in the right inferior parietal lobule and the right inferior temporal gyrus. In males, this effect was absent. Assuming a model that poor sleep quality leads to GMV changes, women with poor sleep quality in the nonclinical population may be more at risk to develop cognitive deficits especially with regard to memory consolidation, as well as later dementia. Future studies of sleep quality and dysfunction should consider “sex” as an important variable influencing neurobiological mechanisms of sleep and its disorders.
Funding
Data collection and sharing for this project was provided by the MGH-USC Human Connectome Project (HCP; principal investigators: Bruce Rosen, MD, PhD, Arthur W. Toga, PhD, Van J. Weeden, MD). HCP funding was provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute of Mental Health (NIMH), and the National Institute of Neurological Disorders and Stroke (NINDS). The Laboratory of Neuro Imaging at the University of Southern California disseminates HCP data.
Disclosure Statement
Financial disclosure: None.
Nonfinancial disclosure: None.
Acknowledgments
We wish to thank two anonymous reviewers for helpful commentaries on the manuscript.
References
- 1. Krystal AD, et al. . Measuring sleep quality. Sleep Med. 2008;9(Suppl 1):S10–S17. [DOI] [PubMed] [Google Scholar]
- 2. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 3. Mollayeva T, et al. . The Pittsburgh Sleep Quality Index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev. 2016;25:52–73. [DOI] [PubMed] [Google Scholar]
- 4. Buysse DJ, et al. . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 5. Morin CM, et al. . The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2): 10.1053/smrv.2002.0186 [DOI] [PubMed] [Google Scholar]
- 7. Suh S, et al. . Sex differences in insomnia: from epidemiology and etiology to intervention. Curr Psychiatry Rep. 2018;20(9):69. [DOI] [PubMed] [Google Scholar]
- 8. Bixler EO, et al. . Women sleep objectively better than men and the sleep of young women is more resilient to external stressors: effects of age and menopause. J Sleep Res. 2009;18(2):221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lindberg E, et al. . Sleep disturbances in a young adult population: can gender differences be explained by differences in psychological status? Sleep. 1997;20(6):381–387. [DOI] [PubMed] [Google Scholar]
- 10. Nowakowski S, et al. . Cognitive behavioral therapy for insomnia and women’s health: sex as a biological variable. Sleep Med Clin. 2019;14(2):185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Unruh ML, et al. . Subjective and objective sleep quality and aging in the sleep heart health study. J Am Geriatr Soc. 2008;56(7):1218–1227. [DOI] [PubMed] [Google Scholar]
- 12. Pengo MF, et al. . Sleep in women across the life span. Chest. 2018;154(1):196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang J, et al. . Emergence of sex differences in insomnia symptoms in adolescents: a large-scale school-based study. Sleep. 2016;39(8):1563–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manber R, et al. . Sex, steroids, and sleep: a review. Sleep. 1999;22(5):540–555. [PubMed] [Google Scholar]
- 15. Mong JA, et al. . Sex differences in sleep: impact of biological sex and sex steroids. Philos Trans R Soc Lond B Biol Sci. 2016;371(1688):20150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang B, et al. . Sex differences in insomnia: a meta-analysis. Sleep. 2006;29(1):85–93. [DOI] [PubMed] [Google Scholar]
- 17. Fatima Y, et al. . Exploring gender difference in sleep quality of young adults: findings from a large population study. Clin Med Res. 2016;14(3–4):138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baglioni C, et al. . Sleep and mental disorders: a meta-analysis of polysomnographic research. Psychol Bull. 2016;142(9):969–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buysse DJ, et al. . Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31(4):473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang J, et al. . Insomnia, sleep quality, pain, and somatic symptoms: sex differences and shared genetic components. Pain. 2012;153(3):666–673. [DOI] [PubMed] [Google Scholar]
- 21. Zhang J, et al. . A community-based study on the association between insomnia and hypothalamic-pituitary-adrenal axis: sex and pubertal influences. J Clin Endocrinol Metab. 2014;99(6):2277–2287. [DOI] [PubMed] [Google Scholar]
- 22. Sexton CE, et al. . Poor sleep quality is associated with increased cortical atrophy in community-dwelling adults. Neurology. 2014;83(11):967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fjell AM, et al. . Self-reported sleep relates to hippocampal atrophy across the adult lifespan—results from the Lifebrain consortium. Sleep. November 2019. doi: 10.1093/sleep/zsz280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Altena E, et al. . Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol Psychiatry. 2010;67(2):182–185. [DOI] [PubMed] [Google Scholar]
- 25. Joo EY, et al. . Hippocampal substructural vulnerability to sleep disturbance and cognitive impairment in patients with chronic primary insomnia: magnetic resonance imaging morphometry. Sleep. 2014;37(7):1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Riemann D, et al. . Chronic insomnia and MRI-measured hippocampal volumes: a pilot study. Sleep. 2007;30(8):955–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Winkelman JW, et al. . Increased rostral anterior cingulate cortex volume in chronic primary insomnia. Sleep. 2013;36(7):991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu S, et al. . Gray matter hypertrophy in primary insomnia: a surface-based morphometric study. Brain Imaging Behav. December 2018. doi: 10.1007/s11682-018-9992-z [DOI] [PubMed] [Google Scholar]
- 29. Spiegelhalder K, et al. . Insomnia does not appear to be associated with substantial structural brain changes. Sleep. 2013;36(5):731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Winkelman JW, et al. . Lack of hippocampal volume differences in primary insomnia and good sleeper controls: an MRI volumetric study at 3 Tesla. Sleep Med. 2010;11(6):576–582. [DOI] [PubMed] [Google Scholar]
- 31. Tahmasian M, et al. . A lack of consistent brain alterations in insomnia disorder: an activation likelihood estimation meta-analysis. Sleep Med Rev. 2018;42:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Riemann D, et al. . The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol. 2015;14(5): 547–558. [DOI] [PubMed] [Google Scholar]
- 33. Vecsey CG, et al. . Sleep deprivation impairs cAMP signalling in the hippocampus. Nature. 2009;461(7267):1122–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Achenbach TM, et al. . DSM-oriented and empirically based approaches to constructing scales from the same item pools. J Clin Child Adolesc Psychol. 2003;32(3):328–340. [DOI] [PubMed] [Google Scholar]
- 35. Achenbach TM, et al. . Manual for the ASEBA Adult Forms & Profiles. Burlington, VT: Research Center for Children, Youth, & Families, University of Vermont; 2003. [Google Scholar]
- 36. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 37. Eickhoff SB, et al. . A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–1335. [DOI] [PubMed] [Google Scholar]
- 38. Eickhoff SB, et al. . Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage. 2006;32(2):570–582. [DOI] [PubMed] [Google Scholar]
- 39. Eickhoff SB, et al. . Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36(3):511–521. [DOI] [PubMed] [Google Scholar]
- 40. Scheperjans F, et al. . Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cereb Cortex. 2008;18(9):2141–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bludau S, et al. . Cytoarchitecture, probability maps and functions of the human frontal pole. Neuroimage. 2014;93(Pt 2):260–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Henssen A, et al. . Cytoarchitecture and probability maps of the human medial orbitofrontal cortex. Cortex. 2016;75:87–112. [DOI] [PubMed] [Google Scholar]
- 43. Palomero-Gallagher N, et al. . Functional organization of human subgenual cortical areas: relationship between architectonical segregation and connectional heterogeneity. Neuroimage. 2015;115:177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stoffers D, et al. . The caudate: a key node in the neuronal network imbalance of insomnia? Brain. 2014;137(Pt 2):610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tzourio-Mazoyer N, et al. . Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. [DOI] [PubMed] [Google Scholar]
- 46. Amunts K, et al. . Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl). 2005;210(5–6):343–352. [DOI] [PubMed] [Google Scholar]
- 47. Baglioni C, et al. . Insomnia disorder is associated with increased amygdala reactivity to insomnia-related stimuli. Sleep. 2014;37(12):1907–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Behrens TE, et al. . Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6(7):750–757. [DOI] [PubMed] [Google Scholar]
- 49. Van Essen DC, et al. ; WU-Minn HCP Consortium The WU-Minn Human Connectome Project: an overview. Neuroimage. 2013;80:62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xie L, et al. . Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zajacova A, et al. . Reliability of self-rated health in US adults. Am J Epidemiol. 2011;174(8):977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bailey M, et al. . Sex differences in circadian timing systems: implications for disease. Front Neuroendocrinol. 2014;35(1):111–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Carrier J, et al. . Sex differences in age-related changes in the sleep-wake cycle. Front Neuroendocrinol. 2017;47:66–85. [DOI] [PubMed] [Google Scholar]
- 54. Baker FC, et al. . Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007;8(6):613–622. [DOI] [PubMed] [Google Scholar]
- 55. Manber R, et al. . Sleep and the menstrual cycle. Health Psychol. 1997;16(3):209–214. [DOI] [PubMed] [Google Scholar]
- 56. Prince TM, et al. . Sleep deprivation during a specific 3-hour time window post-training impairs hippocampal synaptic plasticity and memory. Neurobiol Learn Mem. 2014;109:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tartar JL, et al. . Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23(10):2739–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Takeuchi H, et al. . Shorter sleep duration and better sleep quality are associated with greater tissue density in the brain. Sci Rep. 2018;8(1):5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Diekelmann S, et al. . The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev. 2009;13(5):309–321. [DOI] [PubMed] [Google Scholar]
- 60. Kondratova AA, et al. . The circadian clock and pathology of the ageing brain. Nat Rev Neurosci. 2012;13(5):325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Riemann D, et al. . The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14(1):19–31. [DOI] [PubMed] [Google Scholar]
- 62. Draganski B, et al. . Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427(6972):311–312. [DOI] [PubMed] [Google Scholar]
- 63. Gaser C, et al. . Brain structures differ between musicians and non-musicians. J Neurosci. 2003;23(27):9240–9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Maguire EA, et al. . Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000;97(8):4398–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hänggi J, et al. . The architecture of the chess player’s brain. Neuropsychologia. 2014;62:152–162. [DOI] [PubMed] [Google Scholar]
- 66. Igelström KM, et al. . The inferior parietal lobule and temporoparietal junction: a network perspective. Neuropsychologia. 2017;105:70–83. [DOI] [PubMed] [Google Scholar]
- 67. Riemann D, et al. . REM sleep instability—a new pathway for insomnia? Pharmacopsychiatry. 2012;45(5):167–176. [DOI] [PubMed] [Google Scholar]
- 68. Cheng W, et al. . Functional connectivities in the brain that mediate the association between depressive problems and sleep quality. JAMA Psychiatry. 2018;75(10):1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Goldstein-Piekarski AN, et al. . Integrating sleep, neuroimaging, and computational approaches for precision psychiatry. Neuropsychopharmacology. 2020;45(1):192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hinz A, et al. . Sleep quality in the general population: psychometric properties of the pittsburgh sleep quality index, derived from a German community sample of 9284 people. Sleep Med. 2017;30:57–63. [DOI] [PubMed] [Google Scholar]