Abstract
Thyrotropin-releasing hormone (TRH) is produced by the hypothalamus but most brain TRH is located elsewhere where it acts as a neuromodulator. TRH-positive neurons project to the hypoglossal motoneuron pool where TRH receptor RNA shows a high degree of differential expression compared with the rest of the brain. Strategies to modulate hypoglossal motor activity are of physiological and clinical interest given the potential for pharmacotherapy for obstructive sleep apnea (OSA), a common and serious respiratory disorder. Here, we identified the effects on tongue motor activity of TRH and a specific analog (taltirelin) applied locally to the hypoglossal motoneuron pool and systemically in vivo. Studies were performed under isoflurane anesthesia and across sleep–wake states in rats. In anesthetized rats, microperfusion of TRH (n = 8) or taltirelin (n = 9) into the hypoglossal motoneuron pool caused dose-dependent increases in tonic and phasic tongue motor activity (both p < 0.001). However, the motor responses to TRH were biphasic, being significantly larger “early” in the response versus at the end of the intervention (p ≤ 0.022). In contrast, responses to taltirelin were similar “early” versus “late” (p ≥ 0.107); i.e. once elicited, the motor responses to taltirelin were sustained and maintained. In freely behaving conscious rats (n = 10), microperfusion of 10 μM taltirelin into the hypoglossal motoneuron pool increased tonic and phasic tongue motor activity in non-rapid-eye-movement (REM) sleep (p ≤ 0.038). Intraperitoneal injection of taltirelin (1 mg/kg, n = 16 rats) also increased tonic tongue motor activity across sleep–wake states (p = 0.010). These findings inform the studies in humans to identify the potential beneficial effects of taltirelin for breathing during sleep and OSA.
Keywords: sleep, upper airway, genioglossus, obstructive sleep apnea, thyrotropin-releasing hormone, taltirelin, animal model
Statement of Significance.
The hypoglossal motor nucleus (HMN) innervates the tongue musculature. Strategies to modulate its activity may lead to novel pharmacotherapies for obstructive sleep apnea (OSA). We first identified that both thyrotropin-releasing hormone (TRH) and its analog taltirelin at the HMN increased tongue motor activity in anesthetized rats. Notably, however, whilst the motor responses to taltirelin were sustained and maintained, the responses to TRH were biphasic, with an early activation followed by a decline. In sleeping rats, taltirelin at the HMN increased tongue motor activity most consistently and significantly in non-rapid-eye-movement (REM) sleep. Intraperitoneal injections of taltirelin increased tongue motor activity across sleep–wake states. These preclinical findings support studies of the potential beneficial effects of taltirelin for breathing during sleep and OSA.
Introduction
Obstructive sleep apnea (OSA) is a common and serious clinical problem. Recent prevalence estimates for moderate to severe sleep-disordered breathing in the United States are 10%–17% for 30–70-year-old men and 3%–9% for women [1]. OSA is ultimately caused by the closure of the air passage in the throat in sleep due to relaxation of the tongue muscles whose activity normally keeps the airway open [2]. The hypoglossal motoneuron pool is the source of motor output to the tongue, and strategies to modulate its activity may lead to identification, development, and testing of new pharmacological treatments for OSA [3, 4].
Thyrotropin-releasing hormone (TRH) is produced by the hypothalamus and acts at the anterior pituitary to release thyrotropin-stimulating hormone (TSH). Approximately two-thirds of brain TRH, however, is located outside the hypothalamus [5, 6] where it acts as a neuromodulator to influence motor behavior and cognition [7, 8]. Rodent cranial and spinal motoneurons express dense TRH-binding sites, and TRH exhibits direct excitatory effects on those motoneurons in vitro [7, 9–14]. Studies in vivo, however, are lacking.
Anatomically, TRH-positive neurons project to the hypoglossal motoneuron pool [7, 14, 15], and TRH receptor RNA shows a high degree of differential expression at the hypoglossal motoneuron pool, being 6.3‐fold higher compared with the rest of the brain [4]. Accordingly, it is significant to identify and characterize the effects of TRH (or selected analogs) on upper airway motor activity in vivo. In the present study, we first identify and characterize the effects of microperfusion of TRH into the hypoglossal motoneuron pool on tongue muscle activity.
Although it is of physiological significance to characterize the effects on motor activity of TRH at a central motoneuron pool in vivo, it is also important to note that TRH itself is generally a poor drug candidate for potential therapeutic effects due to its short half-life (<10 minutes) by reason of rapid degradation [8, 13, 16], low intestinal and central nervous system permeability, and endocrine side effects [8]. As a result, there has been interest in developing TRH analogs that are more stable, brain-penetrating, and selective for non-endocrine actions, such as taltirelin [8, 16]. Taltirelin is the first TRH analog approved for human use, in the treatment of neurodegenerative disease [8]. In the present study, we also characterize the effects of taltirelin at the hypoglossal motoneuron pool on tongue muscle activity in vivo and test the hypothesis that the motor-activating effects of taltirelin are sustained and maintained unlike with TRH. These initial studies identifying and characterizing the effects of microperfusion of TRH and taltirelin into the hypoglossal motoneuron pool on respiratory motor activities were performed in isoflurane-anesthetized rats.
Given the growing significance of identifying and testing new pharmacological targets to increase tongue motor activity for potential OSA pharmacotherapy [3, 4], we also performed additional studies in freely behaving rats across natural sleep–wake states. Here, we tested the hypothesis that the sustained activating effects on tongue muscle activity of taltirelin locally delivered to the hypoglossal motoneuron pool are significantly affected by the prevailing sleep–wake state. We also systemically administered taltirelin to identify the effects on respiratory muscle activities and sleep–wake behavior.
Our data identify that TRH and a clinically relevant analog, taltirelin, at the hypoglossal motoneuron pool increase tongue muscle activity in vivo. These activation responses to taltirelin at the hypoglossal motoneuron motor pool are sustained and maintained unlike with TRH and also persist in sleep. Responses to the systemic administration of taltirelin further identify the stimulating effects on tongue muscle activity. These findings inform future studies in humans to identify the potential beneficial effects of taltirelin for breathing during sleep and OSA.
Methods
Ethical approval
Procedures conformed to the recommendations of the Canadian Council on Animal Care and the University of Toronto Animal Care Committee approved the protocols. Adult male Wistar rats (Charles River) were housed individually, maintained on a 12/12-hour light/dark cycle (lights on at 0700 hours), and had free access to food and water. The numbers used for each individual study and protocol are listed below.
Study 1: experiments under general anesthesia
The first set of experiments was performed under general anesthesia to identify and characterize the effects of TRH and its analog, taltirelin, at the hypoglossal motoneuron pool on tongue muscle activity and other respiratory variables.
Animal preparation
Twenty-four rats (mean body weight = 356.3 ± 3.7 [SEM] g, range, 320–380 g) were studied under general anesthesia induced and maintained with isoflurane (typically 2.5%, range: 1.7%–2.7%) sufficient to abolish the corneal blink and pedal withdrawal reflexes and provide stable breathing throughout. For the duration of the experiments, the rats also spontaneously breathed 50% oxygen (balance nitrogen) and were kept warm with a heating pad (T-Pump Model #TP500, Gaymar Industries, Inc., Orchard Park, NY, USA). The rats were positioned supine and bipolar stainless-steel electrodes (AS636, Cooner Wire, Chatsworth, CA, USA) were implanted onto the side of the diaphragm as it meets the abdominal wall to record diaphragm muscle activity. Two platinum needle electrodes (Astro-Med Inc., Grass Instrument Division, West Warwick, RI, USA) were also inserted into the tongue musculature via a per-oral approach to record tongue muscle activity. The rats were then positioned in a stereotaxic frame (Kopf model 962, Tujunga, CA, USA) using blunt ear bars, and with the snout secured in an anesthetic mask (Kopf model 923).
Manipulations of the hypoglossal motoneuron pool
Microdialysis probes (CX-I-12-01, Eicom, San Diego, CA, USA) were placed through a small hole drilled at the junction of the interparietal and occipital bones. The probes were implanted into the hypoglossal motoneuron pool at the following coordinates: 14.0 ± 0.03 (SEM) mm posterior to bregma (range: 13.6–14.5 mm), 0.05 ± 0.01 mm lateral to the midline (range: 0–0.2 mm), and 10.1 ± 0.08 mm ventral to bregma (range: 9.4–10.8 mm). In each rat, a brief burst of tongue muscle activity was observed when the probe penetrated the motor pool. This burst of tongue muscle activity during probe insertion was transient (<5 minutes), did not affect diaphragm activity or respiratory rate, and was useful as a preliminary indication of probe placement [17]. The rats stabilized for at least 30 minutes before the onset of any interventions.
The microdialysis probes were 200 μm in diameter with a 1 mm membrane length, and a 50,000 Dalton cutoff. The probes were connected to fluorinated ethylene propylene (FEP) Teflon tubing (inside diameter = 0.12 mm) in turn connected to 1.0 mL syringes via a zero dead space switch (Uniswitch, B.A.S. West Lafayette, IN). The probes were continually flushed with artificial cerebrospinal fluid (ACSF) at a flow rate of 2.1 μL/min using a syringe pump and controller (MD-1001 and MD-1020, B.A.S. West Lafayette, IN). The lag time for fluid to travel to the tip of the probe at this flow rate was 3 minutes 30 seconds. The ACSF was made fresh each day for each experiment.
Recordings and protocols
The electrophysiological signals were amplified and filtered (Super-Z head-stage amplifiers and BMA-400 amplifiers/filters, CWE Inc., Ardmore, PA). The electroencephalogram (EEG) was filtered between 1 and 100 Hz and the tongue and diaphragm electromyograms (EMGs) between 100 and 1000 Hz. The tongue and diaphragm signals were recorded at the same amplification across all experiments. The electrocardiogram was removed from the diaphragm signal using an electronic blanker (Model SB-1, CWE Inc.). The moving time averages (MTA) of the tongue and diaphragm EMGs were also obtained with a time constant of 100 ms. The signals were digitized at a sampling rate of 2000 Hz using a data acquisition system (CED 1401 and Spike 2 software, version 6, Cambridge Electronic Design Ltd., Cambridge, UK). The following protocols were performed:
Protocol 1a—Responses to TRH at the hypoglossal motoneuron pool
In eight rats, all signals were monitored during control microdialysis perfusion of ACSF into the hypoglossal motoneuron pool (i.e. baseline control condition) followed by microperfusion of TRH (0.001, 0.01, 0.1, 1, and 10 μM, formula weight (FW): 362, Sigma-Aldrich, St. Louis, MO, USA). Each TRH intervention lasted 30 minutes.
Protocol 1b—ACSF time controls at the hypoglossal motoneuron pool
To next examine if any of the observed changes in tongue muscle activity, or other variables, were caused by effects of time per se, i.e. independent of the drug interventions, further experiments were performed in which repeated switches to perfusion of ACSF into the hypoglossal motoneuron pool were performed (i.e. “sham interventions”) over the same time course as the drug interventions (n = 7 rats).
Protocol 1c—Responses to taltirelin at the hypoglossal motoneuron pool
In nine rats, signals were also recorded during control microdialysis perfusion of ACSF into the hypoglossal motoneuron pool (i.e. baseline control condition) followed by microperfusion of taltirelin (TRH analog, 0.1, 1, and 10 μM, FW: 423, Tocris Bioscience, Minneapolis, MN, USA). Each intervention lasted 30 minutes. In five of these rats, the protocol was extended after washout of the 10-μM taltirelin. After washout with ACSF in these rats, a dose of 1 μM taltirelin was then applied continuously for 2 hours to identify if the activation response was sustained.
Analyses
Initial experiments showed that there was a characteristic tongue muscle response to microperfusion of TRH into the hypoglossal motoneuron pool. This response was characterized by a large initial (“early”) response that typically subsequently declined over the course of the intervention (see Results). For each agent delivered to the hypoglossal motoneuron pool, measurements were, therefore, taken over 1-minute periods: (1) at the time of the initial tongue muscle response following application of TRH (the “early” response) and (2) in the last minute of the 30-minute TRH intervention (the “late” response). For those interventions at the hypoglossal motoneuron pool in which there was no response to TRH as the dose was too low (see Results), measurements were also made over 1-minute periods that corresponded to the same average time that the “early” measurements were made at the responsive doses in the same rat. Measurements were also made in the last minute of the 30-minute interventions (i.e. corresponding to the “late” responses). For the time–control interventions with continued ACSF at the hypoglossal motoneuron pool, measurements were also made over 1-minute periods that corresponded to the same average times that the “early” measurements were made in the TRH experiments and the last minute of each intervention. Likewise, for the taltirelin experiments, measurements were also made over 1-minute periods similar to the TRH protocol.
Breath-by-breath measurements of tongue and diaphragm activities were calculated and averaged in consecutive 5-second bins [18, 19]. All values were written in a spreadsheet and then matched to the corresponding intervention at the hypoglossal motoneuron pool to provide a grand mean for each variable, for each intervention, in each rat. The EMGs were analyzed from the respective moving average signal (above electrical zero) and were quantified in arbitrary units. Electrical zero was the voltage recorded with the amplifier inputs grounded. Tongue EMG was quantified as mean tonic activity (i.e. minimal activity in expiration) and within-breath phasic activity (i.e. peak inspiratory activity—tonic activity). Mean diaphragm EMG amplitudes (i.e. phasic respiratory diaphragm activity) and respiratory rates were also calculated [18, 19].
Study 2: experiments across natural sleep–wake states
The second set of experiments was performed in behaving animals to test the hypothesis that the sustained activating effects on tongue muscle activity of taltirelin at the hypoglossal motoneuron pool depended upon prevailing sleep–wake states (protocol 2a). The effects of systemically administered taltirelin on respiratory muscle activities and sleep–wake states were also identified in an additional protocol (protocol 2b).
Anesthesia and surgical procedures
Experiments were performed on 26 rats (mean body weight = 355.8 ± 7.4 g, range: 280–430 g). General anesthesia was induced by inhaled isoflurane (3%–4%) with the animal in an induction chamber and anesthesia was then maintained via a mask placed over the snout (2.2%–2.8% isoflurane). Oxygen was administered to the inspired air (50% oxygen, balance air) throughout the surgery. The rats were also given buprenorphine (1 mg/kg, subcutaneous) and meloxicam (2 mg/kg) for analgesia. Effective anesthesia was judged by abolition of the pedal withdrawal and corneal blink reflexes. During surgery, body temperature was maintained with a water pump and heating pad (T/Pump-Heat Therapy System, Gaymar, Orchard Park, NY, USA).
With the rats placed supine, the ventral surface of the tongue muscle was exposed via a submental incision and dissection of the overlying geniohyoid and mylohyoid muscles. Two insulated, multistranded stainless-steel wires (AS631; Cooner Wire, Chatsworth, CA, USA) were implanted bilaterally into the tongue and secured with sutures. We have shown in previous experiments that tongue muscle activity is markedly decreased, and almost abolished, after section of the medial branches of the hypoglossal nerve, showing that recordings were predominantly from the genioglossus with these electrode placements [17]. To record diaphragm EMG activity, two insulated, multistranded stainless-steel wires (AS636: Cooner Wire) were then sutured onto the costal diaphragm via an abdominal approach. The size, configuration, and placement of the tongue and diaphragm electrodes were consistent across experiments. To further ensure adequate electrode placements during surgery, both the tongue and diaphragm signals were monitored on loudspeaker (AM8 Audio Amplifier, Grass) to document respiratory-related activity. The tongue and diaphragm wires were tunneled subcutaneously to a small incision on the skull and the submental and abdominal incisions were then closed with absorbable sutures.
The rats were then placed in a stereotaxic apparatus (Kopf Model 962, Tujunga, CA, USA) with blunt ear bars. The rats were then implanted with EEG and neck EMG electrodes for subsequent determination of sleep–wake states as previously described [19, 20]. The EEG was recorded via two stainless-steel screws (1.5 mm diameter) attached to insulated wires (30 gauge) positioned on the skull approximately 2 mm anterior and 2 mm to the right of bregma, and 3 mm posterior and 2 mm to the left of bregma, respectively. The reference electrode was placed approximately 5 mm posterior and 3 mm to the left of bregma. Two insulated, multistranded stainless-steel wires were also sutured onto the dorsal neck (trapezius) muscles to record neck muscle activity. Microdialysis guides (CXG-8, Eicom) were targeted stereotaxically 4 mm above the hypoglossal motoneuron pool at the following coordinates: 14.0 ± 0.05 mm posterior to bregma (range: 13.8–14.2 mm), 0.08 ± 0.03 mm lateral to the midline (range: 0–0.3 mm), and 5.8 ± 0.06 mm ventral to bregma (range: 5.5–6.0 mm) [19]. An internal cannula (CXD-8, Eicom) was placed inside the guide to keep it patent until the experiments.
At the end of surgery, the electrodes were connected to pins inserted into a miniature plug (STC-89PI-220ABS, Carleton University, Ottawa, ON, Canada). The plug and microdialysis guides were then fixed to the skull with dental acrylic and anchor screws. After surgery, the rats were transferred to a clean cage and kept warm under a heating lamp until full recovery as judged by normal motor activity, drinking, and eating. The rats were given soft food for the first day after surgery. The rats were then housed individually and recovered for a minimum of 7 days (range: 7–10 days) before the experiments were performed.
Recordings and protocols
For recordings, a lightweight shielded cable was connected to the plug on the rat’s head. The cable was attached to a counterbalanced swivel that permitted free movement. The recording environment consisted of a large open-topped bowl (Rodent Bowl, MD-1514, BAS) housed within an electrically shielded and soundproofed cubicle (EPC-010, BRS/LVE), with the animals free from any disturbance and supplied with fresh bedding, food, and water. A video camera inside the cubicle allowed for continuous monitoring. For habituation, the rats were connected to the cable and swivel apparatus the day before the experiments, typically in the late afternoon from 1700 to 1800 hours. At that time, the internal cannula was removed from the guide, and the microdialysis probe was inserted (CX-I-12-01, Eicom). The probe projected 4 mm from the tip of the guide and, therefore, targeted to the hypoglossal motoneuron pool.
The probes were connected to FEP Teflon tubing (inside diameter, 0.12 mm) with this tubing connected to 1.0 mL syringes via a zero dead space switch (Uniswitch, BAS). The probes were perfused with freshly made ACSF at a flow rate of 0.5 μL/min overnight (this flow rate was changed to 2.1 μL/min the morning of the experiments and was maintained at that rate thereafter). The composition of the ACSF was as follows (in mM): 125 NaCl, 3 KCl, 2 CaCl2, 1 MgSO4, 25 NaHCO3, and 30 glucose. The ACSF was warmed to 37°C and bubbled with CO2 to a pH of 7.4. It is important to note that fluid was not injected into the tissue; rather, the semipermeable membrane at the tip of the microdialysis probe permits passive diffusion only [19].
The electrical signals were amplified and filtered (Super-Z head-stage amplifiers and BMA-400 amplifiers/filters, CWE Inc.). The EEG was filtered between 1 and 100 Hz, whereas the neck, tongue, and diaphragm EMGs were filtered between 100 and 1000 Hz. The electrocardiogram was removed from the diaphragm EMG using an electronic blanker (Model SB-1, CWE Inc.). The MTA of the neck and tongue EMGs (time constant = 200 ms) and the diaphragm EMGs (time constant = 100 ms) were also obtained (Model MA 821, CWE Inc.) as previously described [18, 19]. The raw EEG, tongue and diaphragm signals, and the MTA of the tongue, diaphragm, and neck EMGs were digitized and recorded on the computer (Spike 2 software, 1401 interface, CED Ltd, Cambridge, UK). Two protocols were performed:
Protocol 2a—Responses to taltirelin at the hypoglossal motoneuron pool across sleep–wake states
The experiments began at 0900 hours. In 10 rats, signals were recorded during microperfusion of ACSF into the hypoglossal motoneuron pool (i.e. control condition) for at least 2 hours across the naturally occurring sleep–wake states (i.e. 0900–1100 hours). After this time, the perfusion medium was switched to 1 μM taltirelin, which was also maintained for 2 hours (1100–1300 hours), followed by 10 μM taltirelin for the same period (1300–1500 hours). Data were included for analysis 30 minutes after the switch to each perfusion medium (i.e. over 1.5-h periods for each condition).
Protocol 2b—Responses to systemic administration of taltirelin across sleep–wake states
The experiments began at 0900 hours. In 16 rats, signals were recorded for at least 2 hours (i.e. 0900–1100 hours) following intraperitoneal injection of taltirelin (1 mg/kg). This dose was chosen for systemic administration based on the precedent of previous studies in rats and mice where these doses are well tolerated and exert measurable effects on behavioral outcome measures, such as locomotor activity and antinociception [16, 20, 21]. This time period for analysis was chosen based on previous studies identifying that if a physiological effect of taltirelin were to be observed in the present study, then this time frame would also be able to capture such a response [16, 20, 21]. The responses to systemic taltirelin were compared with the data collected in the same rat over the same time period (i.e. 0900–1100 hours) following intraperitoneal injection of the vehicle alone (0.9% saline). The order of these experiments was balanced, and repeated interventions in the same rat were separated by at least 72 hours. Data were included for analysis 30 minutes after the intraperitoneal injections.
Data analysis
Sleep–wake states were identified visually from the neck EMG and the EEG signals using standard criteria, with data analyses performed as previously described [18, 19]. In summary, measurements of muscle activities within the identified sleep–wake states were made during all periods of wakefulness (>30 seconds in duration), non-rapid-eye-movement (REM) sleep (>60 seconds duration), and REM sleep (>30 seconds duration). Data were included in the analyses of respiratory activity only if they were obtained during such unequivocal and clearly defined states. Periods of wakefulness without any overt body movements (quiet wakefulness) are typically shorter in duration than periods of uninterrupted non-REM sleep, and there are typically fewer periods of REM sleep compared with these other states, which accounts for why periods >30 seconds were analyzed in quiet wakefulness and REM sleep as opposed to >60 seconds in non-REM sleep. Data obtained during transitional states (e.g. drowsiness, arousals from sleep, and transitions from non-REM to REM sleep) were not included in the analyses. Periods of REM sleep without transient muscle activations, i.e. “twitches” (tonic REM sleep), and REM sleep periods with transient muscle activations (phasic REM sleep) were also identified as previously described [19, 20].
As for the experiments under anesthesia in study 1, breath-by-breath measurements of tongue and diaphragm activities were calculated and averaged in consecutive 5-second bins as previously described [18, 19]. Mean neck muscle activity was also calculated [18, 19]. The EEG was also analyzed as previously described [18, 19]. The EEG was sampled by computer at 500 Hz then analyzed on overlapping segments of 1,024 samples, windowed using a raised cosine (Hamming) function and subjected to a fast Fourier transform to yield the power spectrum [18, 19]. The window was advanced in steps of 512 samples, and the mean power spectrum of the EEG signal for each 5-second epoch was calculated. The power contained within six frequency bands was recorded as absolute power and as a percentage of the total power of the signal. The band limits were δ 2 (0.5–2 Hz), δ 1 (2–4 Hz), θ (4–7.5 Hz), α (7.5–13.5 Hz), β 1 (13.5–20 Hz), and β 2 (20–30 Hz).
Histology
At the end of the study, the rats were overdosed with isoflurane. The rats were then perfused intracardially with 0.9% saline and 10% formalin and the brain removed and fixed in 10% formalin. The medullary regions were blocked and transferred to a 30% sucrose solution and subsequently cut in 50-μm coronal sections using a cryostat (CM1850, Leica). Sections were mounted and stained with neutral red. The site of the lesion left by the microdialysis probe was localized and placed on a corresponding standard cross section using an atlas of the rat brain [22].
Statistics
The analyses performed for each statistical test are included in the text where appropriate. For all comparisons, differences were considered significant if the null hypothesis was rejected at p < 0.05 using a two-tailed test. Where post hoc comparisons were performed after analysis of variance with repeated measures (ANOVA-RM), an all-pairwise multiple comparison procedure (Holm–Sidak tests) was then used to determine the significant differences between conditions (e.g. ACSF vs. TRH). Analyses were performed using Sigmaplot version 11 (Systat Software Inc., San Jose, CA, USA). Data are presented as means ± SEM unless otherwise indicated.
Results
Study 1: experiments under isoflurane anesthesia
Sites of microdialysis perfusion
Figure 1 shows an example of a lesion site left by a microdialysis probe in the hypoglossal motoneuron pool and also the distribution of microdialysis sites from each of the experiments in each of the three protocols in study 1 (i.e. protocols 1a, 1b, and 1c). The sites of microdialysis perfusion were located within or immediately adjacent to the hypoglossal motoneuron pool in all experiments for each protocol.
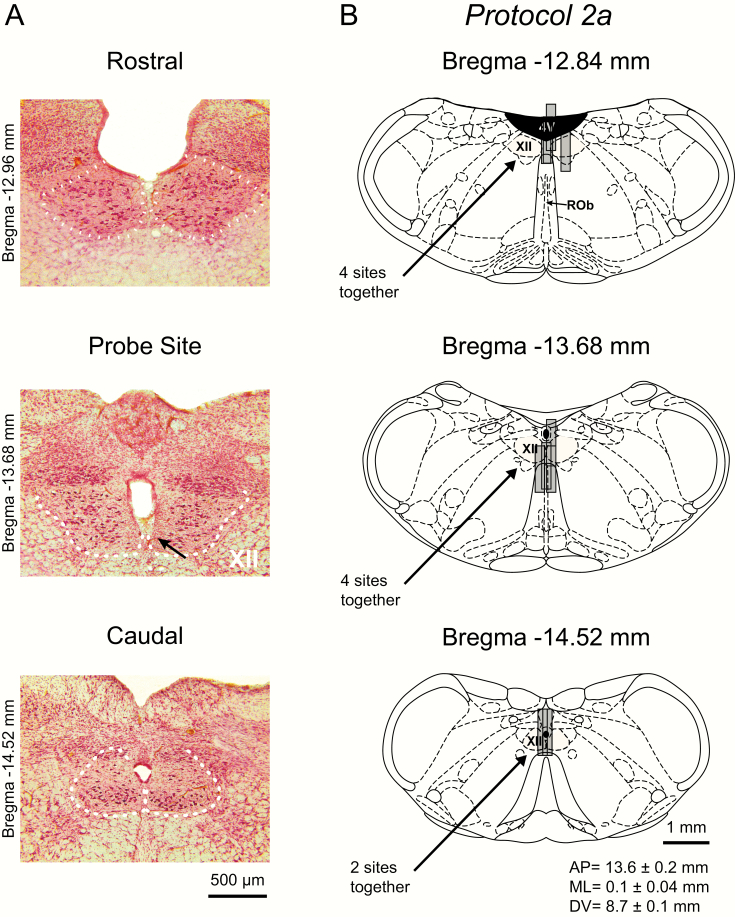
Figure 1.
Example and group data showing the location of the microdialysis probes from all the experiments for each protocol from study 1 in anesthetized rats. The top images (A) show representative histological sections, with the middle section showing the ventral tip of the lesion site left by the microdialysis probe (indicated by the arrow) located immediately adjacent to both hypoglossal motor nuclei; adjacent regions of the HMN (rostral and caudal) are also shown. This figure also includes coronal diagrams of the rat medulla [22] illustrating the distribution of individual microdialysis sites from all rats during microperfusion of the hypoglossal motoneuron pool with TRH (B: protocol 1a, n = 8 rats), ACSF time-control experiments (C: protocol 1b, n = 7 rats), and taltirelin (D: protocol 1c, n = 9 rats). Gray cylinders, drawn to scale, represent the microdialysis probe locations; overlap obscures some of the dialysis sites. The mean stereotaxic coordinates for the ventral tip of the probe sites are given in the anterior–posterior (AP), medial–lateral (ML), and dorsal–ventral (DV) directions for each protocol. The tip of the lesion site left by the microdialysis probe was used to calculate the coordinates after their placement on the corresponding coronal diagrams of the rat medulla. Rob, raphe obscurus; XII, HMN; 4V, fourth ventricle.
Protocol 1a–responses to TRH at the hypoglossal motoneuron pool
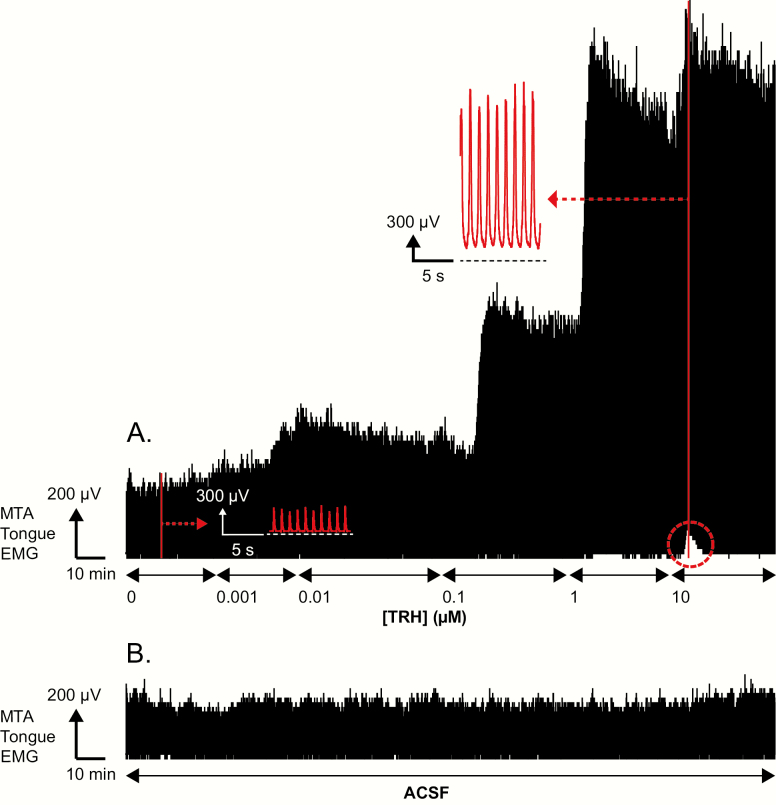
Figure 2 shows an example of the progressive increase in tongue muscle activity with increasing concentration of TRH microperfused into the hypoglossal motoneuron pool over the course of a whole experiment. Note also that the initial response following the application of TRH (“early” response) was higher than the response recorded at the end of the intervention (“late” response). The group data are shown in Figure 3.
Figure 2.
Stimulating effects of TRH at the hypoglossal motoneuron pool on tongue muscle activity. Example representative traces showing the changes in the MTA signal of tongue EMG activity from one rat over the course of a whole experiment for protocol 1a (i.e. TRH, panel A) and protocol 1b (i.e. continuous ACSF throughout, panel B). The baseline of the integrator (i.e. electrical zero) is shown for the MTA signal. (A) The traces show the progressive increase in tongue muscle activity with increasing concentration of TRH microperfused into the hypoglossal motoneuron pool. Note also that following each of the switches to microperfusion of 0.01, 0.1, 1, and 10 μM TRH that the initial peak tongue response following the switch (i.e. the early’ response) was higher than the response recorded at the end of the microperfusion (i.e. the “late” response). In addition, the figure shows that following the application of 10 μM TRH into the hypoglossal motoneuron pool that there was an initial increase in tonic tongue muscle activity as indicated by the increase in baseline signal above electrical zero (see dashed red circle), but that this tonic increase was transient and subsequently declined. The insets show expanded portions of tongue muscle activity recorded during microperfusion of ACSF (i.e. baseline control, 0 mM TRH) and 10 μM TRH into the hypoglossal motoneuron pool at the times indicated by the red vertical lines. For 10 μM TRH note the increased tonic and within-breath phasic tongue muscle activities from the period indicated by the inset. (B) Traces show the absence of changes in the MTA signal of tongue muscle activity recorded from a separate rat over the course of a whole experiment for protocol 1b, i.e. continuous ACSF throughout.
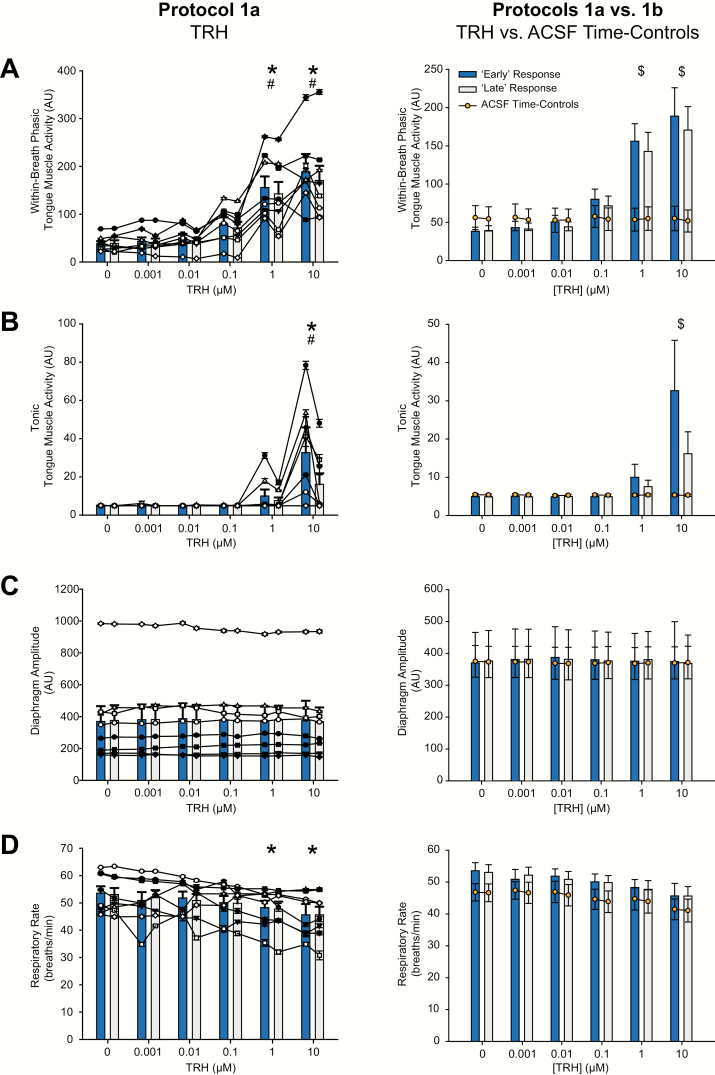
Figure 3.
Group responses to TRH at the hypoglossal motoneuron pool and the ACSF time controls. Group data showing the effects of increasing concentration of TRH microperfused into the hypoglossal motoneuron pool on within-breath phasic (A) and tonic tongue muscle activities (B), diaphragm amplitude (C), and respiratory rate (D). The graphs on the left show individual data derived from each TRH experiment in each rat (n = 8, each symbol represents the mean ± SEM from each animal in protocol 1a) superimposed on the group mean data (vertical bars + SEM). Data are shown for both the “early” (blue bars) and “late” (white bars) responses respectively, i.e. the time of the peak tongue muscle response following application of TRH (or the equivalent average time if no response) and the last minute of the 30-minute interventions (see Methods). The graphs on the right show group mean data comparing protocol 1a to protocol 1b, i.e. the TRH experiments (vertical bars + SEM) to the same periods in the ACSF time-control experiments (n = 7, orange symbol ± SEM) for both “early” and “late” responses, respectively. The symbol * indicates p < 0.05 in protocol 1a for TRH versus the baseline ACSF controls (i.e. [TRH] of 0 μM); the symbol # indicates p < 0.05 in protocol 1a for the “early” versus “late” responses; and the symbol $ indicates p < 0.05 for TRH compared with the same time period in the corresponding ACSF time control (i.e. protocol 1a vs. protocol 1b); AU, arbitrary units. See text for further details.
Within-breath phasic tongue muscle activity
As illustrated in Figures 2 and 3, the statistical analysis identified a significant main effect of TRH dose at the hypoglossal motoneuron pool on the magnitude of within-breath phasic tongue muscle activity (F5,35 = 20.97, p < 0.001, 2-way ANOVA-RM), with increases at 1 and 10 μM compared with the baseline ACSF controls (both t7 > 5.88, both p < 0.001, see symbol “*” in Figure 3A). The magnitude of within-breath phasic tongue muscle activity also differed “early” versus “late” into the responses, respectively, i.e. the time of the peak tongue muscle response following application of TRH versus the last minute of the 30-minute intervention (F1,7 = 8.59, p = 0.022, 2-way ANOVA-RM), with the “early” response to the intervention being larger than the “later” one measured at the end (t7 > 2.93, p = 0.022, see symbol “#” in Figure 3A).
Tonic tongue muscle activity
Figures 2 and 3, and the statistical analysis, also identify a significant main effect of TRH dose at the hypoglossal motoneuron pool on the magnitude of tonic tongue muscle activity (F5,35 = 6.97, p < 0.001, 2-way ANOVA-RM), with increases at 10 μM compared with the baseline ACSF controls (t7 = 4.67, p < 0.001, see symbol “*” in Figure 3B). Figure 3 and the statistical analysis also identified that the magnitude of the tonic tongue muscle activity “early” and “late” into the responses depended on the dose of TRH microperfused into the hypoglossal motoneuron pool; i.e. there was a significant interaction between these two factors (F5,35 = 7.83, p < 0.001, 2-way ANOVA-RM). Post hoc analysis confirmed that the activating effect of TRH on tonic tongue muscle activity was prominent and statistically significant at the time of the peak tongue muscle response following application of 10 μM TRH (the “early” response, t7 = 6.17, p < 0.001), but not in the last minute of the 30-minute 10-μM TRH intervention (the “late” response, t7 = 2.51, p < 0.077) i.e. with 10-μM TRH, there was a decrease in the tonic activation response over the course of the intervention (t7 = 6.77, p < 0.001, see symbol “#” in Figure 3B). This effect is also highlighted in Figure 2A for 10 μM TRH (dashed circle).
Other respiratory variables
There was no change in diaphragm amplitude measured at the different doses of TRH and at the “early” and “late” periods corresponding to the periods that the tongue muscle activities were quantified (Figure 3, F < 1.54, p > 0.20, 2-way ANOVA-RM). It was noted, however, that respiratory rate declined over the course of the experiments (F5,35 = 4.40, p = 0.003, 2-way ANOVA-RM), being significantly decreased at the end of the experiment when 1 and 10 μM TRH were being applied to the hypoglossal motor pool compared with the earlier interventions (both t7 = 2.84, both p < 0.030, see symbol “*” in Figure 3D). To address whether this (or other) effects on the measured variables were due to the TRH interventions at the hypoglossal motoneuron pool per se or more simply due to the effects of time, further analyses were performed including the ACSF time-control experiments; i.e. any changes in the physiological variables in response to TRH in protocol 1a were compared with the equivalent periods in the ACSF time-control experiments performed in protocol 1b (see next section).
Protocol 1b–ACSF time controls at the hypoglossal motoneuron pool
Figure 3 and the statistical analyses showed that there were no changes in tonic or within-breath phasic tongue muscle activities, or diaphragm amplitude, measured over the course of the ACSF time-control experiments (Figure 3, all F < 4.31, all p > 0.08, 2-way ANOVA-RM). However, as in protocol 1a, the respiratory rate also declined over the course of protocol 1b (F5,30 = 4.01, p = 0.007, 2-way ANOVA-RM), being significantly decreased at the time of the sixth application of ACSF to the hypoglossal motor pool at the end of the experiment compared with baseline ACSF at the beginning of the experiment (t6 = 3.53, p = 0.007, Figure 3). Further analyses specifically comparing the changes in respiratory rate in the ACSF time-control experiments (protocol 1b) with the changes in respiratory rate over time in the TRH experiments (protocol 1a) showed that these were indistinguishable for measures made during each intervention and during both “early” and “late” in those interventions (F5,65 = 0.47 and 0.27, p = 0.800 and 0.928, respectively, 2-way ANOVA-RM, Figure 3). These results indicate that the decrease in respiratory rate measured over the course of protocol 1a could be explained by the effects of time per se and were not likely due to TRH as the same change in respiratory rate occurred in the ACSF time-control experiments (protocol 1b).
Comparing tongue muscle responses in the TRH and ACSF time-control experiments also confirmed that tonic and within-breath phasic tongue muscle activities were significantly increased at 1 and 10 μM TRH compared with the corresponding ACSF time controls (each t13 > 3.74, each p < 0.001, see symbol “$” in Figure 3A and B).
Protocol 1c–responses to taltirelin at the hypoglossal motoneuron pool
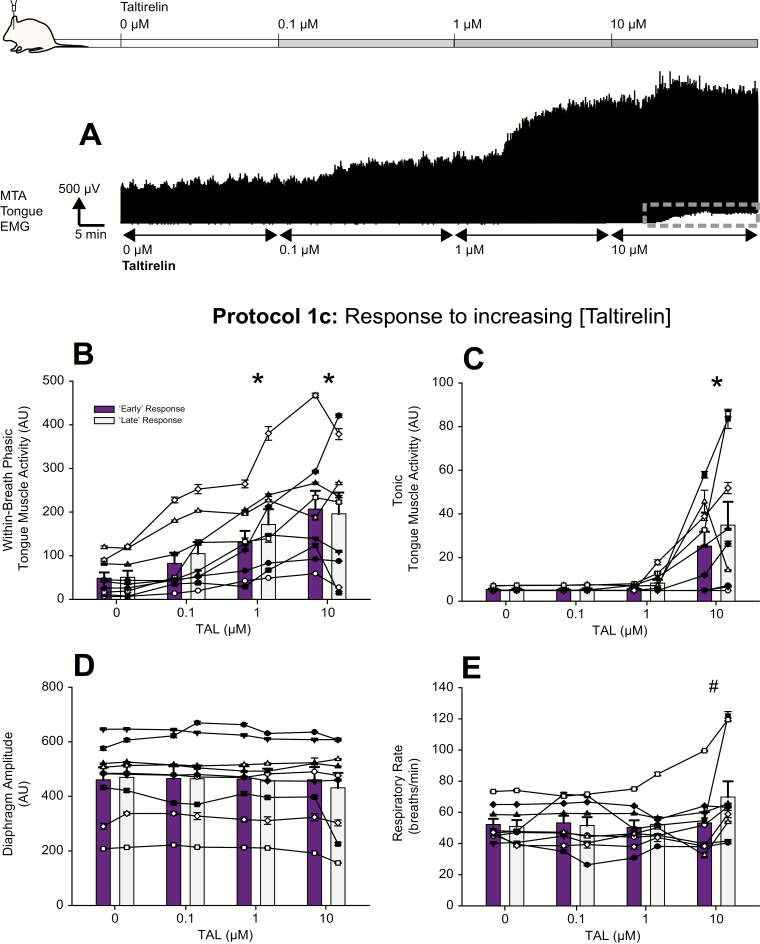
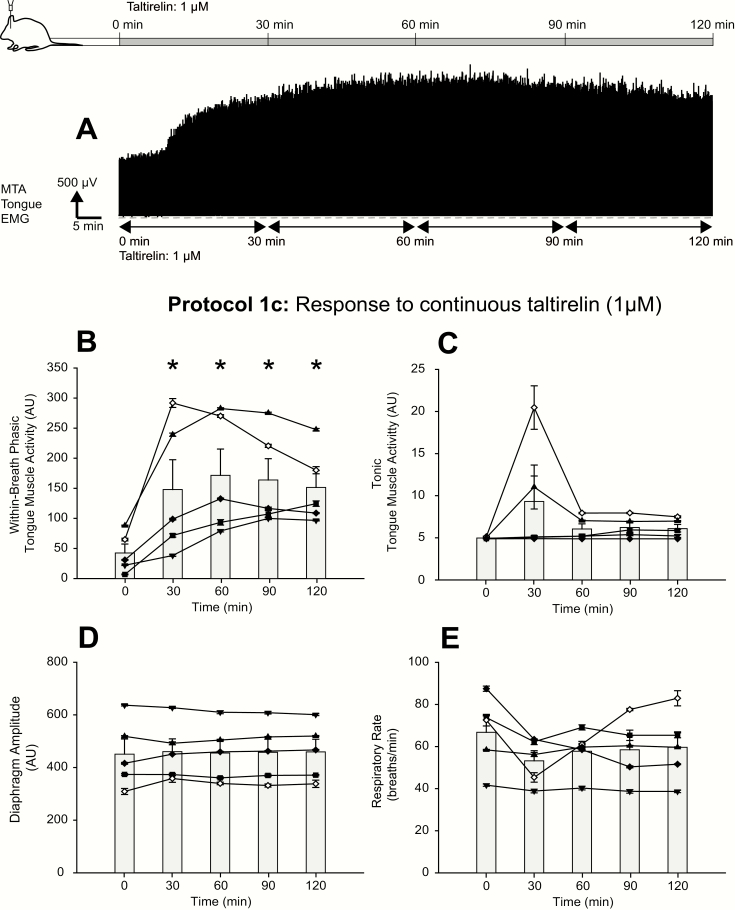
Figures 4 and 5 show examples of the increased tongue muscle activity elicited by microperfusion of taltirelin into the hypoglossal motoneuron pool over the course of an experiment. Note that unlike the characteristic response to TRH (i.e. a large initial response that subsequently declined over the course of the intervention, see Figure 2), the activation response to taltirelin was more sustained over the period of each intervention (Figures 4A and 5A). This more sustained activation was observed in the magnitude of within-breath phasic tongue muscle activity; e.g. at 0.1, 1, and 10 μM taltirelin for the 30-minute interventions in Figure 4A, and with 1 μM taltirelin for the 2-hour interventions in Figure 5A. Levels of tonic tongue muscle activation were also sustained during the 10-μM taltirelin interventions (e.g. compare Figure 2A, dashed red circle, with Figure 4A, dashed gray rectangle).
Figure 4.
Example and group responses to increasing concentrations of taltirelin at the hypoglossal motoneuron pool. (A) Example representative trace showing the changes in the MTA signal of tongue EMG activity from one rat over the course of an experiment for protocol 1c. The traces show the progressive increase in tongue muscle activity with increasing concentration of taltirelin microperfused into the hypoglossal motoneuron pool. Note also that following each of the switches to 0.1, 1, and 10 μM taltirelin that the activation response is sustained over the period of each intervention (unlike for TRH, compare with Figure 2). In addition, the figure shows that the increase in tonic tongue muscle activity with 10 μM taltirelin—as indicated by the increase in baseline signal above electrical zero, see dashed gray rectangle—is also sustained (also unlike for TRH, compare with Figure 2). The group data show the effects of increasing concentration of taltirelin microperfused into the hypoglossal motoneuron pool on within-breath phasic (B) and tonic tongue muscle activities (C), diaphragm amplitude (D), and respiratory rate (E). The graphs show individual data derived from each taltirelin concentration in each rat (n = 9, each symbol represents the mean ± SEM from each animal in protocol 1c) superimposed on the group mean data (vertical bars + SEM). Data are shown for both the “early” (purple bars) and “late” (gray bars) responses, respectively (see Methods). The symbol * indicates p < 0.05 for taltirelin versus the baseline ACSF controls (i.e. [taltirelin] of 0 μM); and the symbol # indicates p < 0.05 in protocol 1c for the “early” versus “late” responses; AU, arbitrary units. See text for further details.
Figure 5.
Example and group responses to sustained application of taltirelin at the hypoglossal motoneuron pool. (A) Example representative trace showing the sustained increase in tongue EMG activity with sustained application of taltirelin (1 μM) microperfused into the hypoglossal motoneuron pool. The group data show the effects of sustained application of taltirelin on within-breath phasic (B) and tonic tongue muscle activities (C), diaphragm amplitude (D), and respiratory rate (E). The graphs show individual data derived from each taltirelin experiment in each rat (n = 5 for these separate prolonged 2-h intervention with 1 μM taltirelin, and each symbol represents the mean ± SEM from each animal in protocol 1c) superimposed on the group mean data (vertical bars + SEM). The symbol * indicates p < 0.05 for taltirelin versus the baseline ACSF controls (i.e. [taltirelin] of 0 μM); AU, arbitrary units. See text for further details.
Figures 4B and C and 5B and C also show group data for the effects of microperfusion of taltirelin into the hypoglossal motoneuron pool on tongue muscle activity for both the sequential 30-minute interventions with 0, 0.1, 1, and 10 μM (n = 9 rats, Figure 4) and the separate prolonged 2-hour intervention with 1 μM taltirelin (n = 5 rats, Figure 5). The analyses of these data are summarized below.
Increasing taltirelin at the hypoglossal motoneuron pool
Statistical analysis identified a significant main effect of taltirelin dose at the hypoglossal motoneuron pool on the magnitude of within-breath phasic tongue muscle activity (F3,24 = 14.33, p < 0.001, 2-way ANOVA-RM), with significant increases at 1 and 10 μM taltirelin compared with the baseline ACSF controls (i.e. 0 μM taltirelin, both t8 > 4.09, both p < 0.001, see symbol “*” in Figure 4B). Unlike the responses to TRH, the magnitude of within-breath phasic tongue muscle activity was not different “early” versus “late” into the responses (F1,8 = 3.30, p = 0.107, 2-way ANOVA-RM), i.e. once elicited, the within-breath phasic tongue muscle response to taltirelin was sustained and maintained.
Statistical analysis also identified a significant main effect of taltirelin dose at the hypoglossal motoneuron pool on the magnitude of tonic tongue muscle activity (F3,24 = 9.43, p < 0.001, 2-way ANOVA-RM), with increases at 10 μM compared with the baseline ACSF controls (i.e. 0 μM taltirelin, t8 = 4.43, p < 0.001, see symbol “*” in Figure 4C). Statistical analysis also identified that (unlike the responses to TRH) the magnitude of tonic tongue muscle activity “early” and “late” into the responses did not depend on the dose of taltirelin microperfused into the hypoglossal motoneuron pool; i.e. there was not a significant interaction between these two factors (F3,24 = 1.46, p = 0.251, 2-way ANOVA-RM). These data show that tonic tongue muscle activity in the presence of taltirelin was not significantly affected by “early” or “late” into the responses, such that once elicited the response was also sustained and maintained.
In contrast to the significant activating effect of taltirelin microperfused into the hypoglossal motoneuron pool on tonic and within-breath phasic tongue muscle activities, there were no effects on diaphragm amplitude (F3,24 = 1.61, p = 0.212, 2-way ANOVA-RM, Figure 4D).
It was noted, however, that respiratory rate did change over the course of the taltirelin experiments (F3,24 = 5.95, p = 0.003, 2-way ANOVA-RM), being significantly increased at the end of the 10-μM taltirelin intervention compared with the beginning (t8 = 4.52, p < 0.001, see symbol “#” in Figure 4E). This stimulating effect of taltirelin was in contrast to the time-related decrease in respiratory rate previously identified and shown in Figure 3 for both TRH and ACSF and analyzed in the previous section (see protocol 1b—ACSF time controls at the hypoglossal motoneuron pool). Further analyses were performed comparing these taltirelin responses to those measured in the ACSF time-control experiments. This analysis identified that the only difference in respiratory rate between these two protocols was an increase in respiratory rate at the end of the 10-μM taltirelin intervention compared with the same period of measurement in the ACSF time-control experiment (t14 = 3.29, p = 0.003 identified by post hoc testing after a significant interaction was identified between the two factors of drug condition [i.e. ACSF or taltirelin] and time period; F3,42 = 9.29, p < 0.001, 2-way ANOVA-RM). This stimulating effect on respiratory rate likely reflects “spillover” of the microperfused taltirelin at this highest dose into neighboring regions of the medulla that can influence breathing frequency (see Discussion).
Prolonged application of taltirelin at the hypoglossal motoneuron pool
In five rats, 1 μM taltirelin was microperfused continuously into the hypoglossal motoneuron pool for 2 hours to identify if the activation response of the tongue muscle was sustained. In four of these rats, the prolonged application of 1 μM taltirelin was applied after the previous sequential 30-minute applications of 0.1, 1, and 10 μM taltirelin. The duration of the washout period with ACSF prior to the 2-hour application of 10 μM taltirelin averaged 90.1 ± 23.0 minutes (range: 33.5–134.5 minutes).
Analysis confirmed a significant effect of this prolonged 1 μM taltirelin application on within-breath phasic tongue muscle activity (F4,16 = 10.52, p < 0.001, 1-way ANOVA-RM), with increases at each of 30, 60, 90, and 120 minutes with 1 μM taltirelin compared with the baseline ACSF controls (i.e. 0 μM taltirelin, all t4 > 4.57, all p < 0.003, see symbol “*” in Figure 5B). The response to taltirelin was sustained and maintained, with no significant differences in the magnitude of within-breath phasic tongue muscle activity between any of the 30, 60, 90, and 120-minute time points (all t4 > 0.15, each p > 0.877).
In contrast to the significant sustained effects on within-breath phasic tongue muscle activity of 1 μM taltirelin microperfused continuously into the hypoglossal motoneuron pool, there was no significant effect of 1 μM taltirelin on tonic tongue muscle activity (F4,16 = 1.87, p = 0.166, 1-way ANOVA-RM, Figure 5C). Likewise, there were no significant effects on diaphragm amplitude or respiratory rate over the course of these experiments (both F4,16 < 1.36, p > 0.293, Figure 5D and E).
Study 2: experiments across natural sleep–wake states
Sites of microdialysis perfusion
Figure 6 shows an example of a lesion site left by a microdialysis probe in the hypoglossal motoneuron pool in the experiments across natural sleep–wake states (i.e. protocol 2a) and also shows the distribution of microdialysis sites from each of the experiments. The sites of microdialysis perfusion were located within or immediately adjacent to the hypoglossal motoneuron pool in these studies.
Figure 6.
Example and group data showing the location of the microdialysis probes from all the experiments for protocol 2a in study 2 in freely behaving rats. The images on the left (A) show representative histological sections, with the middle section showing the ventral tip of the lesion site left by the microdialysis probe (indicated by the arrow); adjacent regions of the HMN (rostral and caudal) are also shown. The images on the right (B) are coronal diagrams of the rat medulla [22] showing the distribution of individual microdialysis sites from all rats (n = 10) and the calculated mean stereotaxic coordinates for the ventral tip of the probe sites. AP, anterior–posterior; DV, dorsal–ventral; ML, medial–lateral; Rob, raphe obscurus; XII, HMN; 4V, fourth ventricle.
Protocol 2a—responses to taltirelin at the hypoglossal motoneuron pool across sleep–wake states
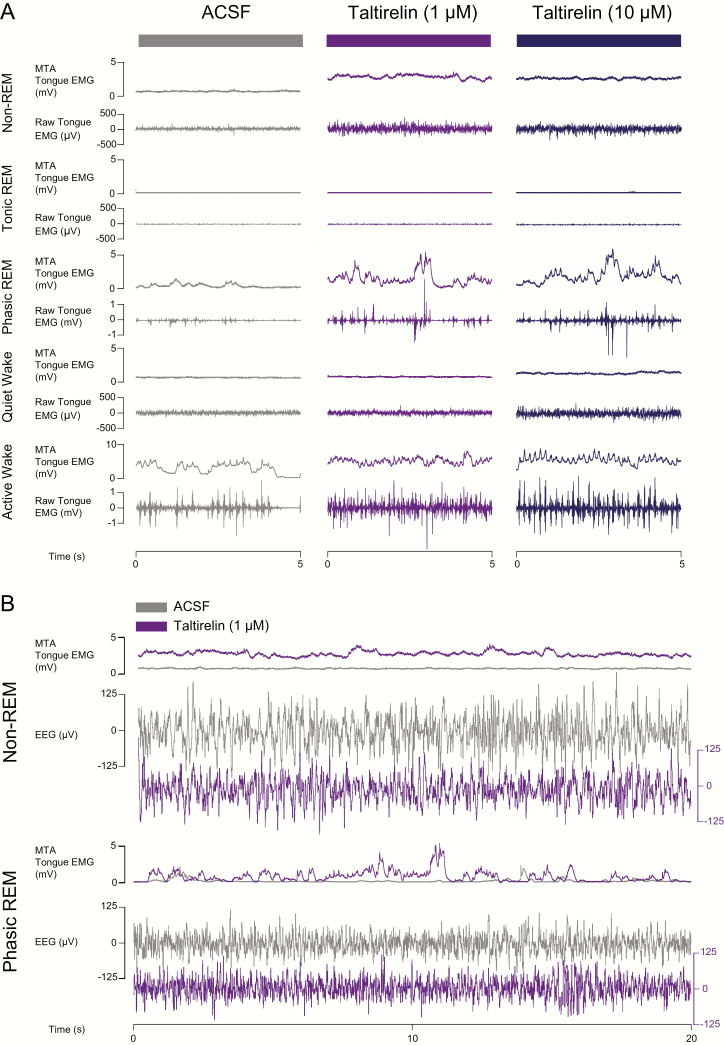
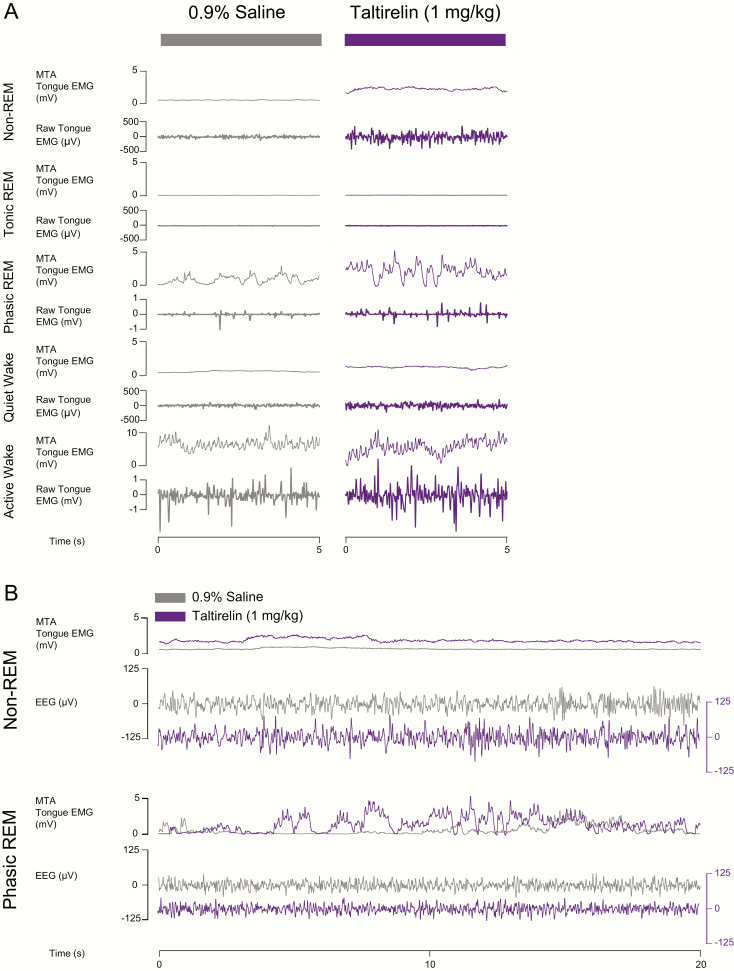
Figure 7 shows an example of the increased tongue muscle activity during microperfusion of taltirelin into the hypoglossal motoneuron pool across sleep and awake states.
Figure 7.
Example traces showing tongue muscle activation in response to microperfusion of taltirelin into the hypoglossal motoneuron pool across sleep–wake states in protocol 2a. (A) Note the increases in tongue muscle activation with taltirelin in non-REM sleep, phasic REM sleep, and wakefulness. (B) A longer period of both non-REM and REM sleep are also shown to illustrate the increased tongue muscle activity elicited by taltirelin (1 μM, purple traces) by superimposition of the tongue muscle activity traces with those recorded during the ACSF (i.e. vehicle) control (gray traces). The EEG traces are not superimposed to show the similarity of the signals with and without taltirelin. See text for further details.
Note the increased tongue muscle activation with taltirelin in the example periods during non-REM sleep, REM sleep, and wakefulness. The averaged individual and group data for all the different sleep–wake epochs recorded in each rat are shown in Figure 8.
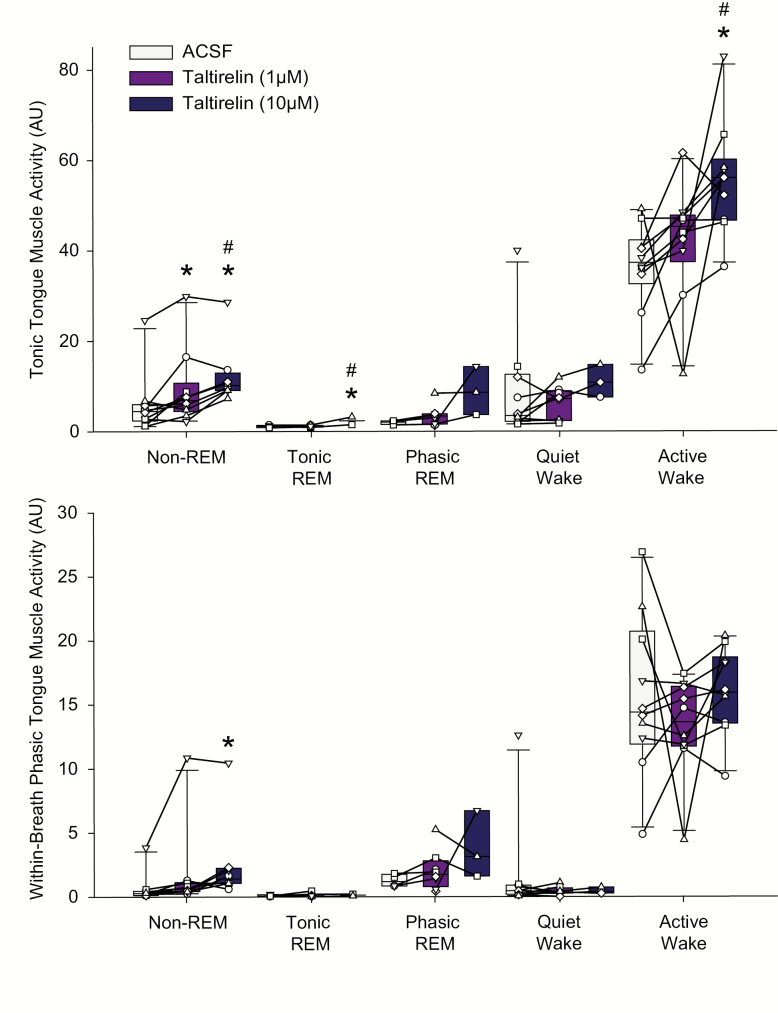
Figure 8.
Individual and average excitatory effects on tongue muscle activity in response to microperfusion of taltirelin into the hypoglossal motoneuron pool across sleep–wake states in protocol 2a. Box (25th and 75th percentiles) and whisker (10th and 90th percentiles) plots show the individual and group (including median) tongue muscle activities recorded over 1.5 hours during microperfusion of ACSF, 1, and 10 μM taltirelin into the hypoglossal motor pool. Data from each animal are superimposed on this plot and represented by a different symbol. Occasionally, an animal may not exhibit a particular state during the recording period with a particular concentration of taltirelin such that a symbol may not be connected with a line (e.g. the rat represented by the symbol ∇ exhibited periods of quiet wakefulness during microperfusion of ACSF into the hypoglossal motoneuron pool but this same state did not occur during the periods with microperfusion of 1 and 10 μM taltirelin). The symbol * indicates p < 0.05 for 1 and/or 10 μM taltirelin versus the baseline ACSF controls (i.e. [taltirelin] of 0 μM). The symbol # indicates p < 0.05 for 10 μM taltirelin versus 1 μM; AU, arbitrary units. See text for further details.
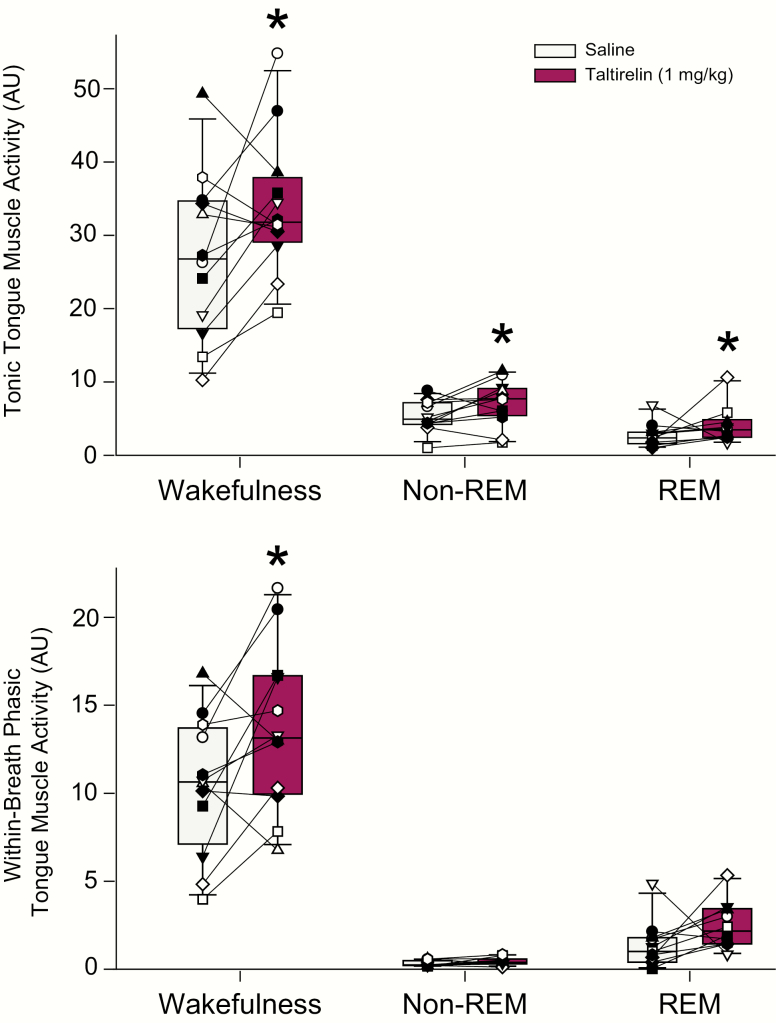
Tonic tongue muscle activity
Figures 7 and 8, and the statistical analysis, identified a significant effect of taltirelin dose at the hypoglossal motoneuron pool on the magnitude of tonic tongue muscle activity in non-REM sleep (F2,16 = 14.36, p < 0.001, 1-way ANOVA-RM). Post hoc analyses identified that both 1 and 10 μM taltirelin increased tonic tongue muscle activity compared with the baseline ACSF controls (p = 0.01 and p < 0.001 for 1 and 10 μM, respectively, see symbol “*” in Figure 8) and that the response to 10 μM taltirelin was significantly larger than with 1 μM (p = 0.037, see symbol “#” in Figure 8).
The other statistically significant effects on tonic tongue muscle activity elicited by microperfusion of taltirelin into the hypoglossal motoneuron pool was observed in tonic REM sleep and active wakefulness for 10 μM taltirelin compared with both 1 μM and the baseline ACSF controls (each p < 0.043 post hoc tests, see symbols “*” and “#” in Figure 8 for tonic REM sleep and active wakefulness).
Within-breath phasic tongue muscle activity
The only statistically significant effect on within-breath phasic tongue muscle activity identified with microperfusion of taltirelin into the hypoglossal motoneuron pool was observed in non-REM sleep (F2,16 = 4.12, p = 0.036), with a significant activating effect at 10 μM compared with the baseline ACSF controls (p = 0.038, see symbol “*” in Figure 8).
Other variables
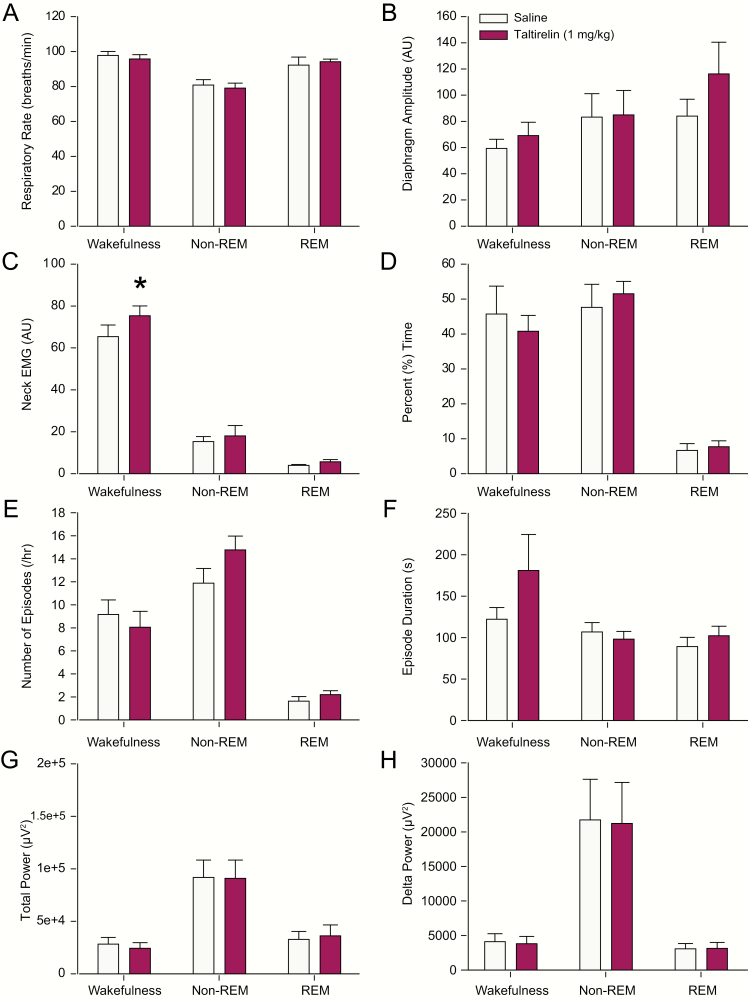
During these experiments in freely behaving rats, there was a consistent stimulating effect on respiratory rate observed during microperfusion of taltirelin into the hypoglossal motoneuron pool across sleep–wake states (Figure 9A). Significant effects were observed in non-REM and REM sleep, and wakefulness (range of F = 22.18–11.53, range of p =< 0.001–0.013), with respiratory rate stimulation observed with 10 μM (and sometimes 1 μM) taltirelin compared with the baseline ACSF controls across these sleep and awake states (range of p < 0.001–0.015, see symbol “*” in Figure 9A). Respiratory rate stimulation was also observed with this higher dose of taltirelin compared with 1 μM across sleep–wake states (each p < 0.019, see symbol “#” in Figure 9A). This stimulating effect of taltirelin on the respiratory rate is consistent with the effect observed at the end of the taltirelin interventions in the anesthetized rats (see symbol “#” in Figure 4E).
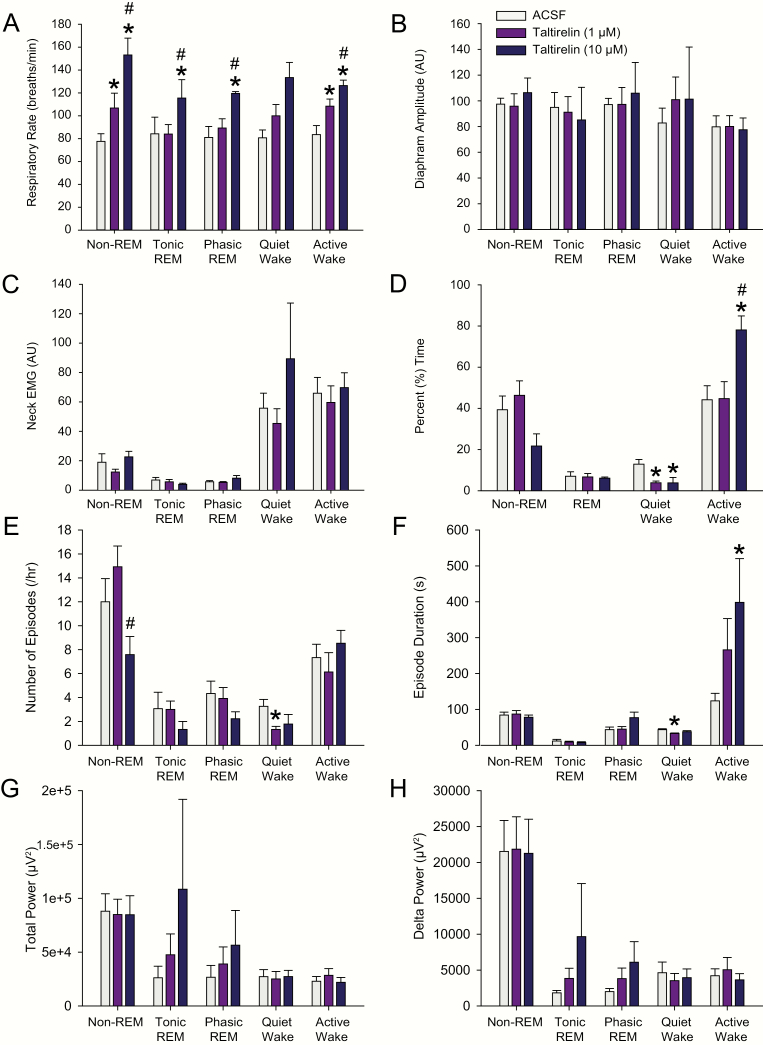
Figure 9.
Group responses to taltirelin at the hypoglossal motoneuron pool across sleep and awake states in protocol 2a. The group data show the effects of microperfusion of ACSF and taltirelin (1 and 10 μM) into the hypoglossal motoneuron pool on respiratory rate (A), diaphragm amplitude (B), neck EMG (C), percent time spent in each sleep–wake state (D), number of sleep–wake episodes per hour (E), sleep–wake episode durations (F), total power in the EEG (G), and delta (0.5–4 Hz) power (H). The symbol * indicates p < 0.05 for 1 and/or 10 μM taltirelin versus the baseline ACSF controls (i.e. [taltirelin] of 0 μM). The symbol # indicates p < 0.05 for 10 μM taltirelin versus 1 μM; AU, arbitrary units. See text for further details.
Unlike the effects on respiratory rate, there were no statistically significant effects on diaphragm amplitude observed during the taltirelin interventions at the hypoglossal motoneuron pool across the sleep–wake states (Figure 9B, range of F = 3.19–0.05, range of p = 0.128–0.952). Likewise, there were also no significant effects on postural (neck) EMG activity during the interventions (Figure 9C, range of F = 5.08–0.06, range of p = 0.063–0.947).
There were no statistically significant effects of the taltirelin interventions on the percentage time spent in REM sleep (p = 0.780, 1-way ANOVA-RM), nor in non-REM sleep (p = 0.057, 1-way ANOVA-RM). For the latter, however, there was a tendency for the percent time spent in non-REM sleep to decrease in the presence of 10 μM taltirelin (Figure 9D) via a decrease in the number of non-REM episodes per hour (see symbol “#” in Figure 9E, p = 0.032 for 10 μM versus 1 μM taltirelin, post hoc test following the identification of a significant treatment effect from the 1-way ANOVA-RM, F = 4.21, p = 0.034). The durations of non-REM sleep episodes, however, were unchanged with taltirelin (Figure 9F, F = 0.22, p = 0.805, 1-way ANOVA-RM).
Perhaps consistent with microperfusion of 10 μM taltirelin eliciting a behavioral response that could explain (or contribute to) the increased respiratory rate identified above, there was an effect of taltirelin on the percentage time spent in active versus quiet wakefulness. Specifically, during microperfusion of taltirelin, there was a statistically significant effect on the percent time spent in active wakefulness (p = 0.004, 1-way ANOVA-RM), with post hoc analysis identifying a significant increase in active wakefulness with 10 μM taltirelin compared with both 1 μM taltirelin and the baseline ACSF controls (both p < 0.012, see symbols “*” and “#” in Figure 9D). The increase in active wakefulness with 10 μM taltirelin was mediated by longer wake durations (see symbol “*” in Figure 9F, p = 0.022 for 10 μM taltirelin vs. ACSF, post hoc test following 1-way ANOVA-RM, F = 4.55, p = 0.025). This increased time spent in active wakefulness during microperfusion of the high dose of taltirelin was mirrored by decreased time spent in periods of quiet wakefulness. Specifically, there was a statistically significant effect on the percent of time spent in quiet wakefulness in the presence of taltirelin (p = 0.002, 1-way ANOVA-RM), with post hoc analysis identifying a significant decrease in quiet wakefulness with 10 μM taltirelin compared with the baseline ACSF controls (p = 0.013, see symbol “*” in Figure 9D), with a similar effect observed with 1 μM taltirelin (p = 0.005). This decrease in quiet wakefulness with taltirelin was mediated by a reduced number of episodes per hour and shorter episode durations that became statistically significant at 1 μM taltirelin compared with ACSF (see symbols “*” in Figure 9E and F, both p < 0.036, post hoc paired t-tests following 1-way ANOVA-RMs, both p = 0.034).
Neither total EEG power (Figure 9G) nor delta power (Figure 9H) was changed during microperfusion of taltirelin into the hypoglossal motoneuron pool across the sleep–wake states (range of F = 2.87–0.30, range of p = 0.108–0.747).
Protocol 2b–responses across sleep–wake states to systemic administration of taltirelin
Tongue muscle activity
Figure 10 shows an example of the increased tongue muscle activity following systemic administration of taltirelin via intraperitoneal injection compared with saline. Note the increased tongue muscle activation with taltirelin in the example periods in non-REM sleep, REM sleep, and wakefulness. The averaged individual and group data across sleep–wake states in each rat are shown in Figure 11.
Figure 10.
Responses across sleep–wake states to systemic administration of taltirelin in protocol 2b. Note the increased tongue muscle activation with systemic administration of taltirelin compared with the saline (vehicle) control in non-REM sleep, REM sleep, and wakefulness in both the shorter and longer periods of each state (A and B, respectively). Superimposition of the tongue muscle activity traces with saline (control, gray traces) and taltirelin (1 mg/kg purple traces) further illustrates the increased tongue muscle activity with systemically administered taltirelin (B). The EEG traces are not superimposed to show the similarity of the signals with and without taltirelin. AU, arbitrary units. See text for further details.
Figure 11.
Individual and average effects on tongue muscle activity in response to systemic administration of taltirelin across sleep–wake states in protocol 2b. Box (25th and 75th percentiles) and whisker (10th and 90th percentiles) plots show the individual and group (including median) tongue muscle activities after intraperitoneal injection of saline or taltirelin (1 mg/kg). Data from each animal are superimposed on this plot and represented by a different symbol. The symbol * indicates p < 0.05; AU, arbitrary units. See text for further details.
When analyzed across each of the five categories of sleep–wake states, i.e. active and quiet wakefulness, non-REM sleep, and tonic and phasic REM sleep, there was no significant effect of systemically administered taltirelin on tonic or within-breath tongue muscle activity (F4,27 < 2.32, p > 0.083). As such, no post hoc tests were performed within these individual five behavioral states. However, one-quarter of the rats did not exhibit all states, typically because one or more periods of quiet or active wakefulness and/or phasic or tonic REM sleep were not present; the pattern of missing data led to the omission of four rats from the analysis above.
For this reason, the wakefulness data (i.e. active and quiet wakefulness) and REM sleep data (phasic and tonic REM) were pooled into single categories for analyses. These data are shown in Figure 11.
Analyses across wakefulness, non-REM sleep, and REM sleep identified a stimulating effect of taltirelin on tonic tongue muscle activity (F1,10 = 9.80, p = 0.010, 2-way ANOVA-RM), that did not depend on sleep–wake state (F2,19 = 3.22, p = 0.063), i.e. there was no interaction such that the activating effect was statistically indistinguishable across states (see symbol “*” in Figure 11, top panel).
There was also a stimulating effect of taltirelin on within-breath tongue muscle activity (F1,10 = 5.87, p = 0.035, 2-way ANOVA-RM) that depended on sleep–wake state (F2,19 = 4.18, p = 0.031). Post hoc analyses identified that systemic administration of taltirelin increased within-breath tongue muscle activity in wakefulness (p < 0.001, see symbol “*” in Figure 11, bottom panel) but not non-REM sleep or wakefulness (p = 0.892 and 0.493, respectively).
Other variables
There was no main effect of systemically administered taltirelin on either respiratory rate or diaphragm amplitude (both F1,10 < 2.07, p > 0.180, 2-way ANOVA-RMs). Likewise, any effect of taltirelin did not depend on the prevailing sleep–wake state, i.e. there was no statistical interaction (both F1,10 < 2.94, p > 0.076, 2-way ANOVA-RMs).
In keeping with a tongue motor-activating effect of systemically administered taltirelin (Figure 11), there was also a stimulating effect on neck muscle activity (F1,10 = 5.80, p = 0.036, 2-way ANOVA-RM). This effect depended on sleep–wake state (F2,19 = 4.40, p = 0.027). Post hoc analyses identified that taltirelin increased neck muscle activity in wakefulness (p < 0.001, see symbol “*” in Figure 12C), but not in non-REM sleep or REM sleep (p = 0.909 and 0.437, respectively).
Figure 12.
Group responses to systemic administration of taltirelin across sleep–wake states in protocol 2b. The group data show effects of intraperitoneal injection of saline or taltirelin (1 mg/kg) on respiratory rate (A), diaphragm amplitude (B), neck EMG (C), percent time spent in each sleep–wake state (D), number of sleep–wake episodes per hour (E), sleep–wake episode durations (F), total power in the EEG (G), and delta (0.5–4 Hz) power (H). The symbol * indicates p < 0.05; AU, arbitrary units. See text for further details.
There was no significant effect of systemically administered taltirelin on the percent time spent in each sleep–wake state (Figure 12D), number of sleep–wake episodes per hour (Figure 12E), sleep–wake episode durations (Figure 12F), total EEG power (Figure 12G), delta power (Figure 12H), or the EEG power in each of the six frequency bands (i.e. δ 2 through β 2, 0.5–30 Hz, range of p = 0.921–0.064).
Discussion
Here, we identified the effects of TRH, and its analog taltirelin, on tongue motor activity and breathing in vivo, following either local microdialysis perfusion into the hypoglossal motoneuron pool or systemic administration (via intraperitoneal injection). The focus was on TRH and taltirelin given the dense projections of TRH-positive neurons to the hypoglossal motoneuron pool [7, 14, 15] and the high degree of differential expression of TRH receptor RNA at this site compared with the rest of the brain [4]. Although the effects of TRH on hypoglossal and other cranial and spinal motoneuron activities have been well studied in vitro [7, 12], in vivo studies of TRH and selected analogs are lacking but are of physiological and translational relevance to human investigation given the potential for OSA pharmacotherapy [4] and/or the potential as a respiratory stimulant to counter drug-induced respiratory depression [23, 24].
Despite the potential translational relevance of identifying the physiological effects of TRH on tongue motor activity and breathing in vivo, TRH is typically not considered viable for therapeutic applications due to rapid degradation, low permeability, and endocrine side effects [8, 16]. As such, more stable and blood–brain barrier penetrating TRH analogs with lesser non-endocrine actions are interest for study, and taltirelin is one such analog that is the focus of this study [8, 16].
Motor-activating effects of TRH versus taltirelin at the hypoglossal motoneuron pool
We first identified and characterized the effects of microperfusion of TRH into the hypoglossal motoneuron pool in vivo, with the expectation that the interventions would elicit increased tongue motor activity. This anticipation was based on in vitro studies identifying that TRH acts directly on hypoglossal motoneurons causing membrane depolarization and decreased input conductance (i.e. increased input resistance) via G protein-mediated responses impacting (at least in part) resting potassium channel conductance [12, 14, 25].
TRH at the hypoglossal motoneuron pool caused dose-dependent increases in tongue motor activity, although a signature feature was that the motor responses to TRH were biphasic, i.e. characterized by an early activation followed by a subsequent decline (Figure 2). Although this bi-phasic motor responses to TRH was not unexpected, it had not previously been identified in vivo. Nevertheless, previous in vitro studies have noted the relatively quick recovery of TRH-induced hypoglossal motoneuron depolarization (e.g. within 8–10 minutes), but with only modest desensitization with repeated applications [12, 13].
Unlike the responses to TRH, however, the activating responses of motoneurons to taltirelin have not previously been investigated, although general effects on motor function such as improvements in ataxia and the flexor reflex have been reported [16]. The new knowledge gained from the present study is of significance given that taltirelin is the only TRH analog approved for human use (in Japan) where it has been trialed as a treatment for adult spinal muscular atrophy [8].
Similar to TRH, taltirelin at the hypoglossal motoneuron pool also caused dose-dependent increases in tongue motor activity. However, a notable difference was that the motor responses to taltirelin were sustained and maintained (Figures 4 and 5) and not biphasic (Figures 2 and 3). This sustained effect with local microperfusion into the hypoglossal motoneuron pool was hypothesized as taltirelin is more stable than TRH with longer-lasting neurobehavioral effects [8, 16].
In the anesthetized rats, in response to both TRH and taltirelin at the hypoglossal motoneuron pool, the predominant activating effect was on the magnitude of within-breath phasic tongue muscle activity, with increases in tonic activity being either not observed, transiently observed, and/or only observed at higher doses (e.g. see Figures 2, 4, and 5). It has also been observed in anesthetized rats that serotonin at the hypoglossal motor nucleus (HMN) also preferentially activates within-breath phasic tongue muscle activity compared with tonic activity [26]. This increase in the phasic component of motor activity is likely best explained by the TRH (and serotonin [26]) receptor-mediated increase in input resistance of hypoglossal motoneurons [12, 14, 25]. This increased input resistance would increase the magnitude of respiratory-related hypoglossal motor output in response to a given incoming respiratory drive potential. The capacity to observe this effect is facilitated in the anesthetized preparation as volatile anesthetics increase the respiratory-related component of tongue muscle activity due to augmentation of hypoglossal premotor respiratory inputs compared with the non-anesthetized awake preparation [27]. The lesser response of tonic activity in the anesthetized rats may also reflect robust homeostatic maintenance of resting membrane potential in vivo [28] and/or that the hypoglossal resting membrane potential remained sufficiently below threshold between inspirations in the presence of general anesthesia such that tonic motor activity was either not observed, transiently observed, and/or only observed at higher doses of TRH and taltirelin if adequately tonically depolarized. This latter suggestion is supported by the observation that increases in tonic tongue motor activity were observed in the awaking and sleeping rats with taltirelin at the hypoglossal motoneuron pool in the absence of general anesthesia (Figures 7 and 8), as also occurs with serotonin [29]. The potential for taltirelin to increase the respiratory-related component of tongue muscle activity and/or tonic activity depending on the balance of the prevailing respiratory-related and/or tonic drives to the hypoglossal motoneuron pool is of relevance to its potential capacity and utility as a pharmacological tool to modulate tongue motor tone in vivo.
There are two TRH receptor (TRHR) genes identified in mammals [30]. In rodents, the endocrine effects of TRH (e.g. release of TSH and prolactin) were thought to be largely mediated via TRHR1 whereas the neuropharmacological effects (e.g. locomotor, respiratory, thermoregulatory, cardiovascular, and arousal) were thought to be largely mediated via TRHR2 [8]. This distinction is questionable, however, not least based on the studies performed in knockout mice for each of TRHR1, TRHR2, and TRHR1/R2 to address the TRH receptor type(s) contributing to the neurobehavioral and autonomic effects of taltirelin, as measured by effects on the arousal responses to sedating agents, antinociception in response to thermal stimuli, and antidepressant activity [31]. The finding of significant effects of taltirelin on each of these behavioral assays in both wild-type mice and TRHR2 knockout mice (i.e. with functional TRHR1), but minimal or no effects in the TRHR1 and TRHR1/R2 knockout mice, supports the view that central nervous effects of taltirelin are mediated primarily/exclusively by TRHR1 [31].
This latter view is also supported by the results of the present study given that taltirelin exerts clear motor-activating effects at the hypoglossal motoneuron pool (e.g. Figures 4 and 5) and that TRHR1, but not TRHR2, are expressed on motoneurons [7]. The more rapid agonist-induced internalization and downregulation/desensitization of TRHR2 compared with TRHR1 [30, 32] may also explain the findings that the motor responses to taltirelin were sustained and maintained and not biphasic as for TRH itself. Although the binding affinities and signaling potencies of taltirelin are lower than for TRH at the human TRH receptor, taltirelin exerts higher intrinsic efficacy and exhibits properties of a super-agonist [33].
TRH versus taltirelin at the hypoglossal motoneuron pool on respiratory network activity
Unlike the tongue motor activation elicited by microperfusion of TRH and taltirelin into the hypoglossal motoneuron pool in the isoflurane-anesthetized rats, there was no effect on the amplitude of diaphragm motor activity (Figures 3–5), indicative of the specificity of the motor-activating effects to the targeted motoneuron pool. In the awake and sleeping animals, there was also no effect of microperfusion of taltirelin into the hypoglossal motoneuron pool on neck muscle activity (Figure 9).
With respect to the rhythmic properties of the respiratory network, in the anesthetized animals, there was a progressive decline in respiratory rate observed over the course of the experiments. However, this decline was indistinguishable for the interventions with TRH and the ACSF time controls, suggesting it could be explained by the effects of time per se and not TRH. In contrast, microperfusion of 10 μM taltirelin into the hypoglossal motoneuron pool in the anesthetized (Figure 4) as well as the awake and sleeping animals (Figure 9) led to consistent and statistically significant increases in respiratory rate. The effects in the anesthetized experiments were noteworthy in that this stimulatory effect on respiratory rate was only observed at the higher does applied at the end of the experiments. This stimulating effect on the respiratory rate may reflect “spillover” of the microperfused taltirelin at this highest dose into neighboring regions of the medulla that can influence breathing frequency. In this respect, nucleus tractus solitarius neurons are immunoreactive for TRH, and TRH increases their rhythmic bursting activity in vitro [34]. Activation of respiratory neurons in the nucleus tractus solitarius in rodents drives breathing through stimulation of respiratory rate [35]. Such an effect may also explain, at least in part, the tachypnea following intracerebroventricular injections of TRH in rodents [36].
In the awake and sleeping animals, microperfusion of taltirelin into the hypoglossal motoneuron pool also led to an increased percent of time spent in active wakefulness with a corresponding relative decrease in quiet wakefulness (Figure 9). Such an “arousing” effect may also have been elicited by potential “spillover” of the microperfused taltirelin into neighboring regions of the brainstem [37] and may have also contributed to the associated increased respiratory rate in the behaving animals. The respiratory-stimulating effect of TRH in humans is also accompanied by central nervous effects indicative of arousal, such as restlessness and augmented vigilance [38].
Overall, systematic identification of the effects of TRH and its analogs on respiratory network activity (to our knowledge) are lacking but are of interest in the present paper given the potential for such interventions as a strategy to stimulate breathing and counter drug-induced respiratory depression [23, 24].
Effects of systemically administered taltirelin
TRH and its analogs have a broad spectrum of neurobehavioral and autonomic effects, including on locomotion and gait, behavior and cognition, arousal, thermoregulation, and nociception [8, 16, 21, 31, 36, 39–44]. Here, we identify the effects on sleep and breathing following systemic (intraperitoneal, 1 mg/kg) administration of taltirelin based on previous rodent studies [16, 20, 21]. To our knowledge, the effects on sleep and breathing have also not been reported previously.
In the present study, the focus of the data analysis following intraperitoneal injection of taltirelin was the first 2 hours because any anticipated responses were expected to be present in this time window for this dose based on pharmacokinetic and behavioral profiles [16, 20, 21]. While providing new knowledge on the effects on sleep, breathing and pharyngeal dilator muscle activity following systemically administered taltirelin, these data should be considered as foundational observations that provide support for more extensive future studies regarding the administration of different doses and testing for longer durations of action. In this context, the present studies are also potentially complicated by the invasive nature of intraperitoneal injections contributing to behavioral arousal, thus influencing the primary measurements of sleep and breathing and potentially masking some of the physiological consequences of the taltirelin. Although intraperitoneal injections are useful as the delivered dose is quantified, other approaches include oral administration [16] or the use of mini-osmotic pumps for drug delivery without the associated animal handling, which can be tested in future studies.
Despite the potential confounds, however, systemically administered taltirelin in the present study led to increased tongue muscle activity across sleep–wake states (Figures 10 and 11) without significant changes in respiratory rate, diaphragm amplitude, percent time spent in the different sleep–wake states, and total EEG power and EEG power in the delta and other frequency bands (Figure 12 and see Results). Interestingly, the lack of effects of systemically administered taltirelin on sleep–wake states or EEG activity was not anticipated given the identified central nervous stimulant properties of taltirelin [8, 23, 31]. Nevertheless, in keeping with a tongue motor-activating effect of systemically administered taltirelin, there was also an activating effect on neck muscle activity, but this was only present during wakefulness and not sleep (Figure 11).
A significant positive outcome relevant to stimulating future work into potential pharmacotherapy for OSA [4] is that the tongue motor-activating effects of taltirelin can be present without significant changes in other components of respiratory activity (respiratory rate and diaphragm amplitude) or indices of sleep–wake regulation and EEG activation. Whether the motor-activating responses persist with repeated applications of taltirelin was not investigated in the present study, but other studies suggest such a maintenance of responses at least over 2 weeks of taltirelin treatment [20]. Such repeatability of responses would also be beneficial clinically. The findings from the present study inform future human investigation to identify the potential beneficial effects of taltirelin for breathing during sleep in the context of OSA.
Compared to TRH, taltirelin is reported to be 10–100 times more potent as a neuro-stimulant and approximately eight times longer-lasting, as opposed to having 5 to 10 times lower endocrine action in releasing TSH [8, 16, 44]. The strong neuro-stimulant effects and comparatively weaker endocrine actions of taltirelin are thought to be due to two interacting factors: (1) taltirelin having low affinity but high stability for TRH receptors in the central nervous system leading to relatively potent and long-lasting neural actions (as opposed to TRH itself having high affinity but low stability leading to weak and short-lived neural effects of TRH) and (2) taltirelin having low affinity for TRH receptors in the pituitary leading to comparably weaker endocrine effects of taltirelin (as opposed to TRH itself which has a high affinity for TRH receptors in the pituitary leading to the stronger endocrine effects) [8, 16, 33]. The enhanced neuro-stimulatory potency of TRH analogs has also been attributed to differences in metabolic stability, i.e. high for analogs such as taltirelin but low for TRH [45]. This effect leads to more of the circulating stable analog being able to penetrate the blood–brain barrier to exert its neural effects, with these effects being enhanced and prolonged (compared with TRH) also due to increased stability [45].
In the present study, the levels of TSH, triiodothyronine (T3), and thyroxine (T4) at different time points were not measured in the different protocols. However, these and other physiological measures are relevant to future studies if the respiratory stimulant effects of systemically administered taltirelin are to be harnessed for human investigation and intervention, e.g. as potential OSA pharmacotherapy. In this respect, oral doses of taltirelin can increase TSH, T3, and T4 but within the normal ranges when applied at 5 mg once a day or 2.5 mg twice a day for 2 weeks, with normal blood pressure, pulse rate, body temperature, electrocardiogram, blood, and urinary examinations [16]. It is unknown if taltirelin altered body temperature in the present study and whether this could have contributed to the increased respiratory rate in addition to the aforementioned effects on components of the respiratory network [34, 35] and increased arousal [37, 38, 44]. However, systemically administered TRH and taltirelin can increase body temperature as well as locomotor activity [16, 44, 46]. In the present study, increases in respiratory rate were observed with local microdialysis perfusion into the hypoglossal motoneuron pool, but typically at the highest does (potentially implicating “spillover” into neighboring brain regions as discussed above). However, no changes in respiratory rate were observed following the systemic injections (Figure 12A).
Overall, it remains to be determined if there is a separation of taltirelin doses that can stimulate upper airway muscle activity with beneficial effects for upper airway mechanics and collapsibility relevant to OSA, within acceptable limits for other potential changes in other physiological variables in humans.
Funding
This work was supported by funds from the Canadian Institutes of Health Research (CIHR, Grant MT-15563 awarded to RLH) and the National Sanitarium Association Innovative Research Program (fund number 00144051 awarded to RLH). WL was supported by the China Scholarship Council. RLH is supported by a Tier I Canada Research Chair in Sleep and Respiratory Neurobiology (fund number 950-229813).
Conflict of interest statement. None declared.
References
- 1. Peppard PE, et al. . Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Remmers JE, et al. . Pathogenesis of upper airway occlusion during sleep. J Appl Physiol Respir Environ Exerc Physiol. 1978;44(6):931–938. [DOI] [PubMed] [Google Scholar]
- 3. White DP. Pharmacologic approaches to the treatment of obstructive sleep apnea. Sleep Med Clin. 2016;11(2):203–212. [DOI] [PubMed] [Google Scholar]
- 4. Horner RL, et al. . A resource of potential drug targets and strategic decision-making for obstructive sleep apnoea pharmacotherapy. Respirology. 2017;22(5):861–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Winokur A, et al. . Thyrotropin-releasing hormone: regional distribution in rat brain. Science. 1974;185(4147):265–267. [DOI] [PubMed] [Google Scholar]
- 6. Hökfelt T, et al. . Distribution of thyrotropin-releasing hormone (TRH) in the central nervous system as revealed with immunohistochemistry. Eur J Pharmacol. 1975;34(2):389–392. [DOI] [PubMed] [Google Scholar]
- 7. Rekling JC, et al. . Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80(2):767–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khomane KS, et al. . Novel thyrotropin-releasing hormone analogs: a patent review. Expert Opin Ther Pat. 2011;21(11):1673–1691. [DOI] [PubMed] [Google Scholar]
- 9. Nicoll RA. Excitatory action of TRH on spinal motoneurones. Nature. 1977;265(5591):242–243. [DOI] [PubMed] [Google Scholar]
- 10. White SR. A comparison of the effects of serotonin, substance P and thyrotropin-releasing hormone on excitability of rat spinal motoneurons in vivo. Brain Res. 1985;335(1):63–70. [DOI] [PubMed] [Google Scholar]
- 11. Rekling JC. Excitatory effects of thyrotropin-releasing hormone (TRH) in hypoglossal motoneurons. Brain Res. 1990;510(1):175–179. [DOI] [PubMed] [Google Scholar]
- 12. Bayliss DA, et al. . Mechanisms underlying excitatory effects of thyrotropin-releasing hormone on rat hypoglossal motoneurons in vitro. J Neurophysiol. 1992;68(5):1733–1745. [DOI] [PubMed] [Google Scholar]
- 13. Bayliss DA, et al. . Effects of thyrotropin-releasing hormone on rat motoneurons are mediated by G proteins. Brain Res. 1994;668(1–2):220–229. [DOI] [PubMed] [Google Scholar]
- 14. Bayliss DA, et al. . Early postnatal development of thyrotropin-releasing hormone (TRH) expression, TRH receptor binding, and TRH responses in neurons of rat brainstem. J Neurosci. 1994;14(2):821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johansson O, et al. . Immunohistochemical support for three putative transmitters in one neuron: coexistence of 5-hydroxytryptamine, substance P- and thyrotropin releasing hormone-like immunoreactivity in medullary neurons projecting to the spinal cord. Neuroscience. 1981;6(10):1857–1881. [DOI] [PubMed] [Google Scholar]
- 16. Kinoshita K, et al. . Taltirelin Hydrate (TA‐0910): An orally active Thyrotropin‐releasing hormone mimetic agent with multiple actions. CNS Drug Rev. 1998;1:25–41. [Google Scholar]
- 17. Morrison JL, et al. . Glycine at hypoglossal motor nucleus: genioglossus activity, CO(2) responses, and the additive effects of GABA. J Appl Physiol (1985). 2002;93(5):1786–1796. [DOI] [PubMed] [Google Scholar]
- 18. Grace KP, et al. . Identification of the mechanism mediating genioglossus muscle suppression in REM sleep. Am J Respir Crit Care Med. 2013;187(3):311–319. [DOI] [PubMed] [Google Scholar]
- 19. Grace KP, et al. . Identification of a pharmacological target for genioglossus reactivation throughout sleep. Sleep. 2014;37(1):41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Asai H, et al. . Lack of behavioral tolerance by repeated treatment with taltirelin hydrate, a thyrotropin-releasing hormone analog, in rats. Pharmacol Biochem Behav. 2005;82(4):646–651. [DOI] [PubMed] [Google Scholar]
- 21. Tanabe M, et al. . The synthetic TRH analogue taltirelin exerts modality-specific antinociceptive effects via distinct descending monoaminergic systems. Br J Pharmacol. 2007;150(4):403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paxinos G, et al. . The Rat Brain in Stereotaxic Coordinates, 7th ed San Diego, CA: Academic Press; 2014. [Google Scholar]
- 23. Dahan A, et al. . Averting opioid-induced respiratory depression without affecting analgesia. Anesthesiology. 2018;128(5):1027–1037. [DOI] [PubMed] [Google Scholar]
- 24. Boghosian JD, et al. . Intravenous and intratracheal thyrotropin releasing hormone and its analog taltirelin reverse opioid-induced respiratory depression in isoflurane anesthetized rats. J Pharmacol Exp Ther. 2018;366(1):105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Talley EM, et al. . TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25(2):399–410. [DOI] [PubMed] [Google Scholar]
- 26. Sood S, et al. . 5-HT at hypoglossal motor nucleus and respiratory control of genioglossus muscle in anesthetized rats. Respir Physiol Neurobiol. 2003;138(2–3):205–221. [DOI] [PubMed] [Google Scholar]
- 27. Roda F, et al. . Effects of anesthetics on hypoglossal nerve discharge and c-Fos expression in brainstem hypoglossal premotor neurons. J Comp Neurol. 2004;468(4):571–586. [DOI] [PubMed] [Google Scholar]
- 28. Brickley SG, et al. . Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409(6816):88–92. [DOI] [PubMed] [Google Scholar]
- 29. Jelev A, et al. . Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J Physiol. 2001;532(Pt 2):467–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun Y, et al. . Thyrotropin-releasing hormone receptors – similarities and differences. J Mol Endocrinol. 2003; 30(2):87–97. [DOI] [PubMed] [Google Scholar]
- 31. Thirunarayanan N, et al. . Thyrotropin-releasing hormone receptor type 1 (TRH-R1), not TRH-R2, primarily mediates taltirelin actions in the CNS of mice. Neuropsychopharmacology. 2013;38(6):950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O’Dowd BF, et al. . TRH-R2 exhibits similar binding and acute signaling but distinct regulation and anatomic distribution compared with TRH-R1. Mol Endocrinol. 2000;14(1):183–193. [DOI] [PubMed] [Google Scholar]
- 33. Thirunarayanan N, et al. . Taltirelin is a superagonist at the human thyrotropin-releasing hormone receptor. Front Endocrinol (Lausanne). 2012;3:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dekin MS, et al. . Thyrotropin-releasing hormone induces rhythmic bursting in neurons of the nucleus tractus solitarius. Science. 1985;229(4708):67–69. [DOI] [PubMed] [Google Scholar]
- 35. Fu C, et al. . Activation of Phox2b-expressing neurons in the nucleus tractus solitarii drives breathing in mice. J Neurosci. 2019;39(15):2837–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Koivusalo F, et al. . The effect of centrally administered TRH on blood pressure, heart rate and ventilation in rat. Acta Physiol Scand. 1979;106(1):83–86. [DOI] [PubMed] [Google Scholar]
- 37. Hedner J, et al. . Respiratory stimulant effects by TRH into the mesencephalic region in the rat. Acta Physiol Scand. 1987;130(1):69–75. [DOI] [PubMed] [Google Scholar]
- 38. Nink M, et al. . Thyrotropin-releasing hormone has stimulatory effects on ventilation in humans. Acta Physiol Scand. 1991;141(3):309–318. [DOI] [PubMed] [Google Scholar]
- 39. Yamamura M, et al. . Pharmacological study of TA-0910, a new thyrotropin-releasing hormone (TRH) analog (IV): effects on experimental memory impairment in mice and rats. Jpn J Pharmacol. 1991;55(2):241–253. [DOI] [PubMed] [Google Scholar]
- 40. Yamamura M, et al. . Pharmacological study of TA-0910, a new thyrotropin-releasing hormone (TRH) analog (III): inhibition of pentobarbital anesthesia. Jpn J Pharmacol. 1991;55(1):69–80. [DOI] [PubMed] [Google Scholar]
- 41. Yamamura M, et al. . Pharmacological study of TA-0910, a new thyrotropin-releasing hormone (TRH) analog (II): involvement of the DA system in the locomotor stimulating action of TA-0910. Jpn J Pharmacol. 1991;55(1):57–68. [DOI] [PubMed] [Google Scholar]
- 42. Faden AI, et al. . Novel TRH analog improves motor and cognitive recovery after traumatic brain injury in rodents. Am J Physiol. 1999;277(4):R1196–R1204. [DOI] [PubMed] [Google Scholar]
- 43. Clarke KA, et al. . Stimulation of locomotion in neonate rats by TRH analogue CG3703. Neuropeptides. 1994; 26(1):71–75. [DOI] [PubMed] [Google Scholar]
- 44. Yamamura M, et al. . Pharmacological study of TA-0910, a new thyrotropin-releasing hormone (TRH) analog, (I): effects on the central nervous system by oral administration. Jpn J Pharmacol. 1990;53(4):451–461. [DOI] [PubMed] [Google Scholar]
- 45. Metcalf G. Regulatory peptides as a source of new drugs–the clinical prospects for analogues of TRH which are resistant to metabolic degradation. Brain Res. 1982;257(3):389–408. [DOI] [PubMed] [Google Scholar]
- 46. Schuhler S, et al. . Thyrotrophin-releasing hormone decreases feeding and increases body temperature, activity and oxygen consumption in Siberian hamsters. J Neuroendocrinol. 2007;19(4):239–249. [DOI] [PubMed] [Google Scholar]