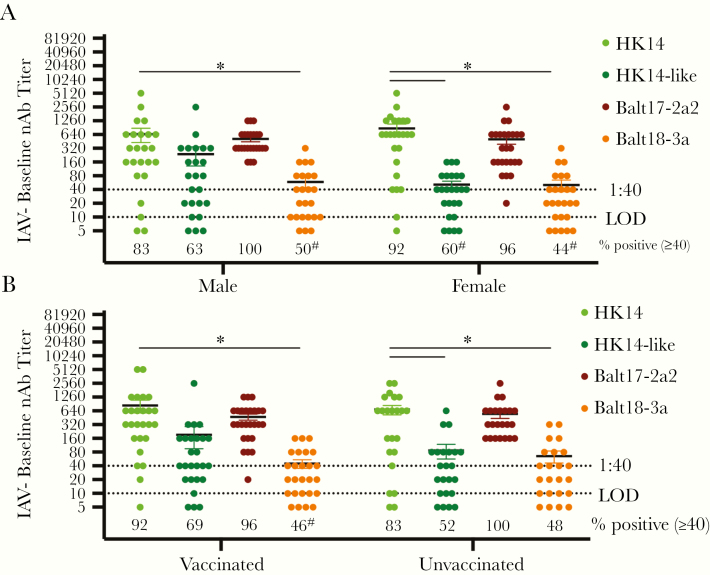
Figure 2.
Among influenza A virus (IAV)-negative patients, neither vaccination nor patient sex affected baseline neutralizing antibody (nAb) titers to the H3N2 vaccine relative to the circulating H3N2 viruses during the 2017–2018 season. A, Baseline serum samples from male and female patients were used to measure nAb titers against the HK14 and HK14-like H3N2 vaccine viruses and the Balt17-2a2 and Balt18-3a circulating viruses. The limit of detection (LOD) is labeled with a stippled line as is the cutoff for seroprotection (1:40). The percentage of patients with nAb titers > 1:40 is indicated below each virus. For both male and female IAV-positive patients, significant differences in the proportion of patients with a > 1:40 nAb titer against the HK14 virus relative to the HK14-like or circulating viruses are indicated with #. B, Baseline serum samples from vaccinated and unvaccinated patients were used to measure nAb titers against the HK14 and HK14-like H3N2 vaccine viruses and the Balt17-2a2 and Balt18-3a circulating viruses during the 2017–2018 influenza season in asymptomatic, IAV-negative patients. Significant differences in nAb titers against the HK14 relative to the HK14-like or circulating H3N2 viruses as well as between IAV-negative and IAV-positive patients for an individual virus are represented with *. For both vaccinated and unvaccinated IAV-negative patients, significant differences in the proportion of patients with a > 1:40 nAb titer against the HK14 virus relative to the HK14-like or circulating viruses are indicated with #.