Important Compound Classes

Title
Heteroaromatic Compounds as Vanin Inhibitors
Patent Publication Number
WO 2020/114943 A1
Publication Date
June 11, 2020
Priority Application
EP 18209721.2
Priority Date
December 3, 2018
Inventors
Fleck, M. T.; Godbout, C.; Koolman, H. F.
Assignee Company
Boehringer Ingelheim International GmbH, Germany
Disease Area
Cancer, inflammatory diseases, and immunomodulatory agent
Biological Target
Vanin
Summary
In humans, three vanin family members, namely vanin-1, vanin-2, and vanin-3, have been described and these are classified as members of the biotinidase branch of the nitrilase superfamily. To date the only known substrate for vanin-1 is pantetheine, and it is believed that vanin-1 acts as the predominant pantetheinase in vivo catalyzing its hydrolysis to produce pantothenic acid (vitamin B5) and cysteamine. These products impact diverse biological processes. Pantothenic acid is a necessary factor in the synthesis of Coenzyme A (CoA), a cofactor involved in many metabolic processes such as fatty acid synthesis and oxidation of pyruvate. The amino-thiol cysteamine, the second product of vanin-1 enzymatic reaction, impacts the cellular redox status.
Increased vanin-1 activity in the gut epithelium has been implicated in promoting tissue damage and inflammation by reducing resistance to oxidative stress in murine models. Homozygous VNN1 knockout (KO) mice lack appreciable levels of cysteamine in blood and tissues and show glutathione-mediated tissue resistance to oxidative stress. Given rodents lack vanin-2, their only source of cysteamine is from vanin-1; therefore, the protective phenotype of the VNN1 KO mouse is attributed to the lack of cysteamine.
In addition, upregulation of vanin-1 activity in the skin and blood has been linked to development and severity of fibrosis in Systemic Sclerosis patients, and elevated levels of vanin-1 have been observed in chronic Juvenile Idiopathic Thrombocytopenia, Psoriasis, and Atopic Dermatitis. Elevated vanin-1 expression and activity are also present and serve as biomarkers for pancreatic cancer associated new-onset diabetes and are also correlated with poor prognosis and response to treatment in colorectal cancer.
The present application describes a series of novel heteroaromatic compounds as inhibitors of vanin and are useful for treatment of cancer and inflammatory diseases and as immunomodulatory agent. The compounds are specifically used for treating Crohn’s disease, ulcerative colitis, atopic dermatitis, systemic sclerosis, Non-Alcoholic Steatohepatitis (NASH), psoriasis, chronic kidney disease, chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis, rheumatoid arthritis, scleroderma, asthma, allergic rhinitis, allergic eczema, juvenile rheumatoid arthritis, juvenile idiopathic arthritis, Hyperlipidemia, colorectal cancer, or pancreatic cancer related new onset diabetes. Further, the application discloses compounds, their preparation, use, pharmaceutical composition, and treatment.
Definitions
n = 1 or 2;
R1, R2, and R3 = independently from each other selected from the group consisting of C1–4-alkyl optionally substituted by hydroxy, CH3O-, CH3–SO2-, phenyl-CH2- optionally substituted by 1 to 3 halogen atoms and 5–6 membered heteroaryl-C1–2-alkyl; or
R2 and R3 together form a 3–6 membered carbocycle or 4–6 membered heterocyclyl containing one heteroatom selected from the group consisting from N and O; or
R1, R2, and R3 together form a bicyclic 5–8 membered carbocycle or a bicyclic 6–8 membered heterocyclyl containing one heteroatom selected from the group consisting from N and O; wherein R1, R2, and R3 = alkyl, cycloalkyl, heteroaryl, and heterocyclyl are optionally substituted by 1 to 3 halogen atoms;
R4 = R4.1R4.2N-, 5–6 membered heteroaryl, NC- or 5–6 membered heterocyclyl; or
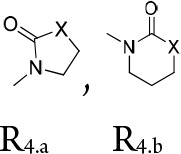
R4 = a group of formula R4.a or R4.b
wherein X = CH2-, -NRx or O; wherein Rx = H or C1–3-alkyl;
R4.a and R4.b = independently from each other are optionally substituted by methyl;
R4.1 = C1–4-alkyl-CO- optionally substituted by 1–3 F atoms; C3–4-cycloalkyl or C1–2-alkoxy, C3–5-cycloalkyl-CO- substituted with R4.1.1 and R4.1.2, 4–6 membered heterocyclyl-CO- substituted with R4.1.3 and R4.1.4, 4–6 membered heterocyclyl-CH2–CO, 5–6 membered heteroaryl-CO- optionally substituted by 1 halogen atom, H3CO- or 1 to 2 methyl, phenyl-CO-substituted with R4.1.5 and R4.1.6, (C1–3-alkyl)(C1–3-alkyl)N-CO- and 5–6 membered heteroaryl;
R4.1.1, R4.1.2 independently from each other are selected from group consisting of H, −CH3, F, CF3, and -CN;
R4.1.3, R4.1.4 independently from each other are selected from group consisting of H, -CH3, F, CF3, and -CN;
R4.1.5, R4.1.6 independently from each other are selected from group consisting of H, -CH3, F, CF3, and -CN;
R4.2 = H, C1–4-alkyl, C3–4-cycloalkyl, C3–4-cycloalkyl-C1–2-alkyl-, and phenyl-C1–2-alkyl; wherein R4.2 = alkyl, cycloalkyl, and phenyl optionally substituted by 1–3 F atoms or one C1–2-alkyl-O-;
R5 = H or C1–2-alkyl; or
R4 and R5 together form 4–6 membered heterocyclyl containing 1 heteroatom selected from group consisting of N and O.
Key Structures
Biological Assay
The human vanin-1 enzymatic assay and human whole blood (HWB) assay was performed. Pantetheinase (vanin) converts pantetheine into pantothenic acid and cysteamine. The HWB assay is applicable to identify vanin inhibitors. The compounds described in this application were tested for their ability to inhibit vanin-1 and human whole blood (HWB). The vanin-1 IC50 (μM) and HWB IC50 (μM) are shown in the following table.
Biological Data
The table below shows representative
compounds were tested for vanin-1 and human whole blood inhibition.
The biological data obtained from testing representative examples
are listed in the following table.
Claims
Total claims: 16
Compound claims: 13
Use of compound claims: 1
Pharmaceutical composition claims: 2
Recent Review Articles
-
1.
Bartucci R.; Salvati A.; Olinga P.; Boersma Y. L.. Int. J. Mol. Sci. 2019, 20, 3891.
-
2.
Schalkwijk J.; Jansen P.. Biochem. Soc. Trans. 2014, 42, 1052.
The author declares no competing financial interest.


