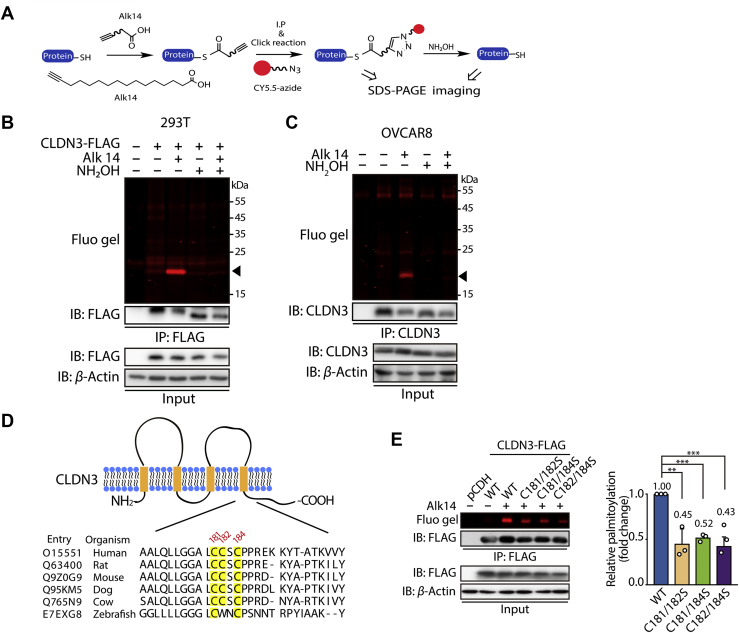
Figure 2.
CLDN3 is S-palmitoylated at multiple cysteine sites. (A) The schematic overview of the Alk-14 metabolic labeling method to study fatty acylation of CLDN3. (B) In-gel fluorescence detection of the S-palmitoylation level of exogenous FLAG-tagged CLDN3 overexpressing in 293T cells by Alk-14 metabolic labeling method with or without NH2OH treatment. Black triangle indicates fluorescent band of S-palmitoylated CLDN3. (C) In-gel fluorescence detection of the S-palmitoylation level of endogenous CLDN3 in OVCAR8 cells by Alk-14 metabolic labeling method with or without NH2OH treatment. Black triangle indicates fluorescent band of S-palmitoylated CLDN3. (D) Schematic representation of CLDN3 structure (top panel) and comparison of amino acid sequences of CLDN3 at the C-terminus in different species (bottom panel). The model depicts the conserved amino acid sequence of CLDN3. CLDN3 alignments for the parts of the intracellular C-terminus domain where the potential palmitoylations occur, including multiple CLDN3 homologs. Shown are human CLDN3 (O15551), rat CLDN3 (Q63400), mouse CLDN3 (Q9Z0G9), dog CLDN3 (Q95KM5), cow CLDN3 (Q765N9) and zebrafish CLDN3 (E7EXG8). Amino acid positions of the potential palmitoylation are indicated above the alignment. (E) In-gel fluorescence shows the S-palmitoylation level of CLDN3 WT, C181/182S, C181/184S and C182/184S overexpressed in 293T cells. Quantification of the fluorescence intensity relative to CLDN3 WT is shown on the right panel. Values with error bars indicate mean ± SD of three biological replicates. (∗∗P < 0.01; ∗∗∗P < 0.001).