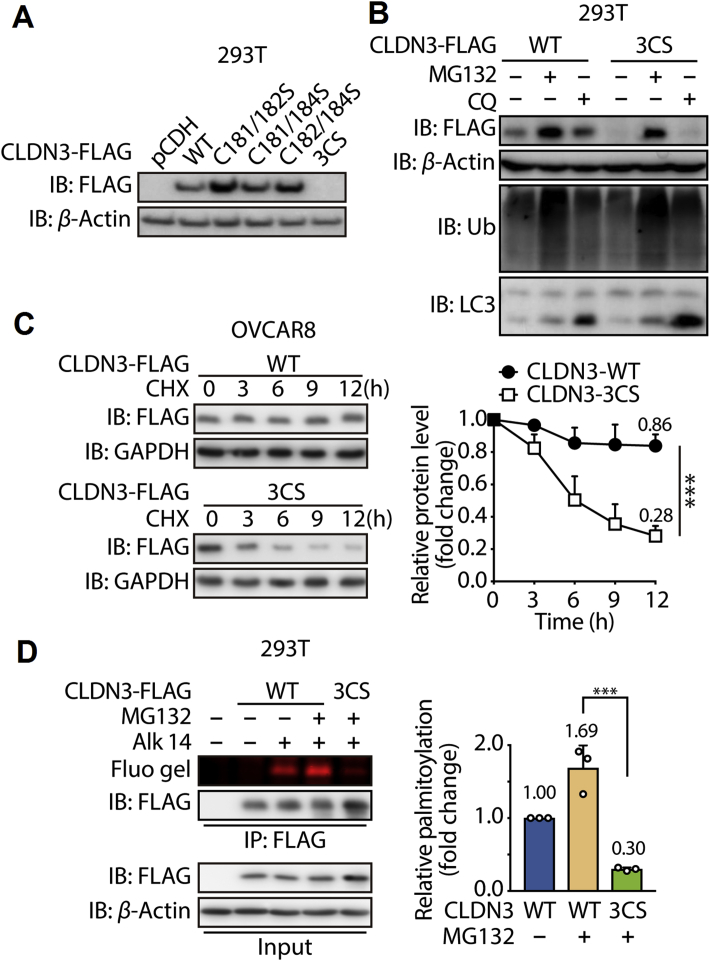
Figure 3.
The S-palmitoylation of CLDN3 is required for its protein stability. (A) Expression of FLAG-tagged CLDN3 wildtype, double cysteine mutants and triple cysteine mutant that transfected in 293T cells was validated by immunoblotting with anti-FLAG antibody. (B) Validation of the degradation pathway for CLDN3 WT and CLDN3 3CS. 293T cells were transfected with FLAG-tagged CLDN3 WT or FLAG-tagged CLDN3 3CS. Each group of cells were treated with MG132 for 8 h or with CQ for 24 h or with DMSO before lysed and tested by immunoblotting. (C) Degradation kinetics curves of CLDN3 WT and CLDN3 3CS. OVCAR8 cells stably expressing FLAG-tagged CLDN3 WT or 3CS mutant were exposed to MG132 for 8 h, followed by the treatment of CHX for 3, 6, 9 and 12 h. Lysates were resolved by immunoblotting. The protein level of CLDN3 WT and CLDN3 3CS with CHX treatment for 0 h is set to 1. Quantification of relative protein level in each group at the indicated time point is shown on the right. Values with error bars indicate mean ± SD of three independent replicates (∗∗∗P < 0.001). (D) In-gel fluorescence shows the S-palmitoylation level of CLDN3 WT and CLDN3 3CS. 293T cells were transfected with FLAG-tagged CLDN3 WT or FLAG-tagged CLDN3 3CS. After the treatment of MG132 and Alk-14, cells were lysed and submitted to immunoprecipitation and immunoblotting. Quantification of fluorescent intensities relative to CLDN3 WT is shown on the right panel. Values with error bars indicate mean ± SD of three independent experiments (∗∗∗P < 0.001).