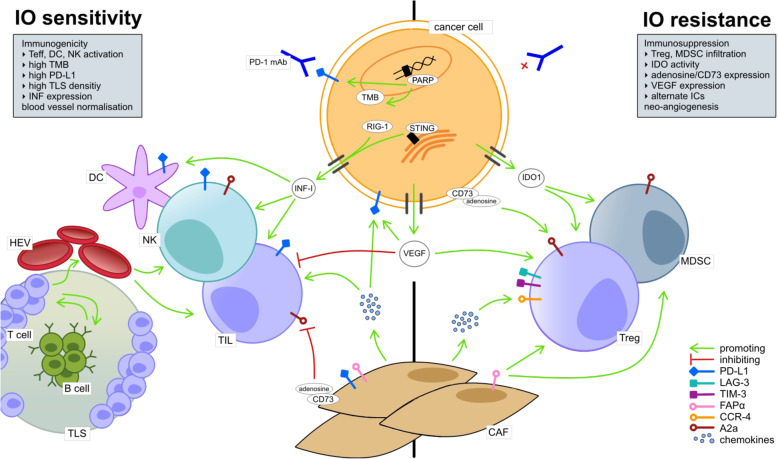
Fig. 1.
Overview of the cellular TME composition and major molecular pathways associated with IO sensitivity (left) and resistance (right). IO sensitivity is depicted by an immunogenic TME, comprising the activation of effector immune cells (e.g. tumor infiltrating lymphocytes (TIL), dendritic cells (CD) and natural killer cells (NK)). Naïve T cells undergo activation and priming in close association to B cells within tertiary lymphoid structures (TLS). T effector cells transmigrate to the stromal TME compartment via high endothelial venules (HEV), tightly regulated by immunomodulatory tumor endothelial cells (TEC; not illustrated) in the HEV endothelium. Cancer cell intrinsic molecular pathways that enhance TME immunogenicity involve interferon type I (IFN I) expression, which is, amongst other stressors, induced by cytosolic RIG-I or by an activated STING pathway. IO sensitivity is enhanced in a TME with high PD-L1 expression by cancer and immune cells. High neo-antigen expression by cancer cells as result of high tumor mutational burden (TMB), e.g. induced by PARP inhibition, enhances TME immunogenicity and IO sensitivity. IO resistance is marked by an immunosuppressive TME and includes, on a cellular basis, infiltration of T regulatory cells (Treg) and myeloid derived suppressor cells (MDSC) as well as M2 macrophages (not shown). CD73 and, thus, adenosine expression by cancer cells or fibroblasts leads to inhibition of TIL and promotion of Treg; CD73 upregulation associates with cancer immune evasion. Also, up-regulation of alternative immune checkpoints e.g. LAG-3 and TIM-1 by immune cells enhances IO resistance. Cancer associated fibroblasts (CAF) depict both immunosuppressive and immunostimulatory functions, e.g. via chemokine release. Upregulation of the chemokine receptor CCR-4 is associated with IO resistance. Vascular endothelial growth factor (VEGF) gets ubiquitously expressed in the TME (not illustrated, see Fig. 2). It has immunosuppressive functions by inhibiting effector immune cells (e.g. TIL, NK, DC), upregulating inhibitory immune checkpoints (e.g. PD-L1) and by promoting Treg and MDSC. Tumor growth promoting neo-angiogenesis (not illustrated) is driven by hypoxia and, thus, VEGF expression